PharmaSources/XiaoyaowanOctober 28, 2021
Tag: COVID-19 , clinical trial , Drugs
With the wide spread of Delta mutant worldwide, the COVID-19 infection situation is worrisome. In addition to vaccination for prevention, drug therapy can alleviate the disease deterioration of patients and improve clinical benefits, which is an important part of the treatment plan for COVID-19.
Recently, the COVID-19 human immunoglobulin (pH4) for intravenous injection developed by Tiantan Biological Products Co., Ltd. has obtained the Drug Clinical Trial Approval Document issued by National Medical Products Administration, and the clinical trials were approved. COVID-19 specific immunity is a specific human immunoglobulin made from the plasma of healthy people immunized with inactivated virus vaccine, which contains highly effective neutralizing antibody against SARS-CoV-2. It is the first in the world. This is another meaningful attempt by domestic bio pharmaceutical companies aimed at the treatment of COVID-19.
With the wide spread of Delta mutant worldwide, the COVID-19 infection situation is worrisome. In addition to vaccination for prevention, drug therapy can alleviate the disease deterioration of patients and improve clinical benefits, which is an important part of the treatment plan for COVID-19.
COVID-19 drugs that have been approved for marketing/emergency use authorization in the world
Up to now, there are not many COVID-19 drugs that have been approved for marketing/emergency use authorization in the world. The drugs approved for marketing are remdesivir of Gilead, Molnupiravir of MSD, REGEN-COV2 of Regeneron/Roche Pharmaceuticals; COVID-19 drugs approved for emergency use authorization are baricitinib of Eli Lilly Pharmaceuticals, Bamlanivimab/Etesevimab of Griffin/Eli Lilly Pharmaceuticals, Sotrovimab of GSK and proxalutamide of Kintor Pharmaceuticals.
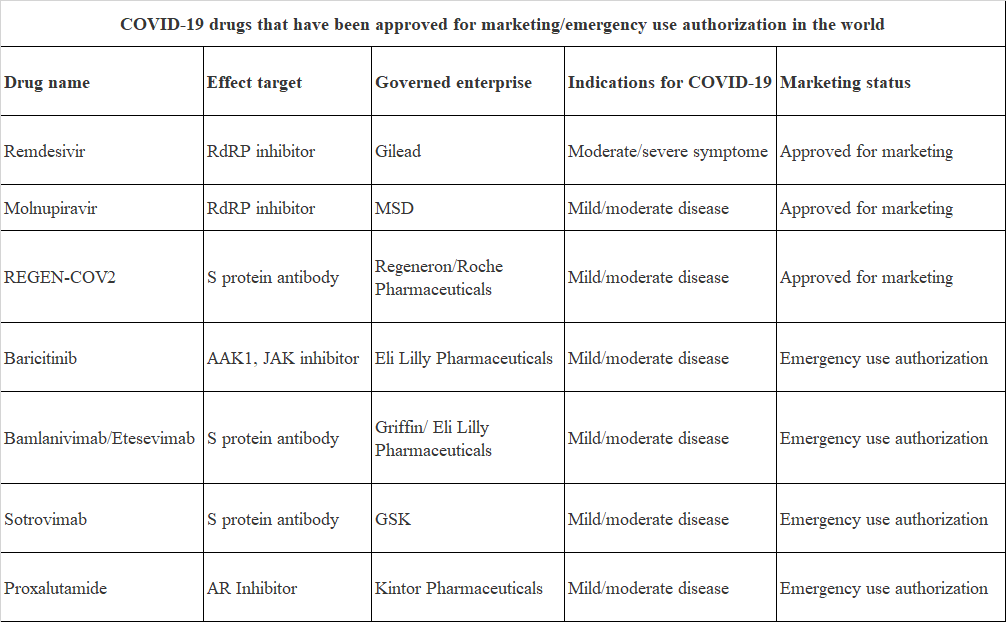
Source: Public data compilation
In terms of drug categories, COVID-19 drugs that have been approved for marketing/ emergency use authorization worldwide cover pharmaceutical chemicals, neutralizing antibodies and macromolecular non-neutralizing antibody drugs.
Pharmaceutical chemicals, remdesivir and Molnupiravir, are RdPR inhibitor, which can be incorporated into newly synthesized viral RNA chain to hinder the replication of viral RNA. Cocktail therapy REGEN-COV2 belongs to S protein antibody, which is aimed at two independent and non-overlapping sites of SARS-CoV-2 S protein receptor binding region, and can reduce the risk of virus mutation and escape, and prevent virus from entering cells. Baricitinib is an AAK1 and JAK inhibitor, which was originally used to treat rheumatoid arthritis. It has the potential activity of blocking SARS-CoV-2 infection and reducing inflammatory reaction by inhibiting JAK pathway. Bamlanivimab/Etesevimab is a neutralizing monoclonal antibody targeting SARS-CoV-2 S protein, which was isolated from the serum of patients in the recuperation period from China and the United States. Sotrovimab is an all-human SARS-CoV-2 monoclonal neutralizing antibody, which can bind with S protein to prevent virus from entering cells of human body. Proxalutamide is an androgen receptor (AR) antagonist, which is used in many AR-related indications. In the treatment of COVID-19, proxalutamide can reduce the gene transcription and translation of ACE2 receptor and TMPRSS2 protease, and block SARS-CoV-2 from entering host cells.
In addition to the above-mentioned COVID-19 drugs which have been approved for marketing/ emergency use authorization, there are currently about 400 under-research COVID-19 drugs in the clinical research stage according to incomplete statistics. Among them, many domestic under-research COVID-19 drugs have entered Phase III clinical stage, and the following four kinds of under-research drugs are worth special attention.
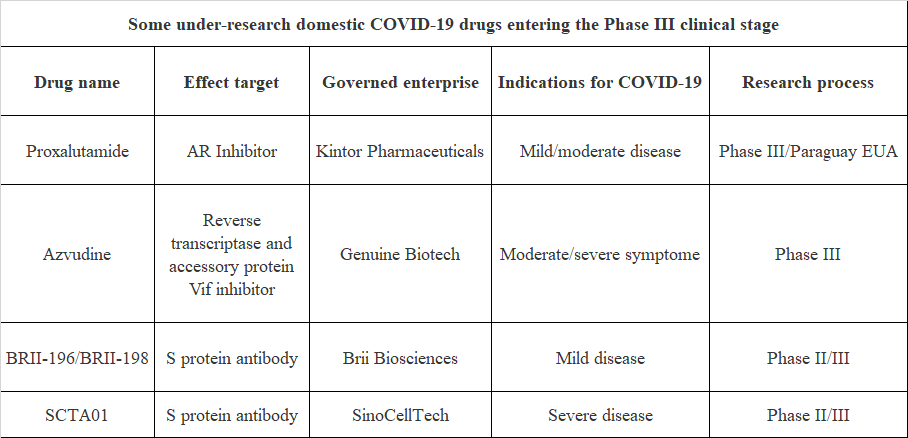
Proxalutamide is a new generation of androgen receptor (AR) antagonist independently developed by Kintor Pharmaceuticals, which is used in a variety of AR-related indications.
For the treatment of COVID-19, proxalutamide can reduce the gene transcription and translation of ACE2 receptor and TMPRSS2 protease and block SARS-CoV-2 from entering host cells. In addition, proxalutamide can also activate Nrf2 pathway to induce the expression of cell protection genes and antioxidant genes, preventing the further deterioration of moderate and severe diseases.
At present, proxalutamide has obtained the first emergency use authorization in Paraguay, and Phase III clinical trials for mild, moderate and severe diseases of COVID-19 have been carried out simultaneously.
As a dual-target inhibitor of reverse transcriptase and accessory protein Vif, Azvudine is the first oral anti-HIV drug with independent intellectual property rights in China, which was approved for adult HIV-1 infected patients in July this year.
Last October, the sub-journal of Nature - Signal Transformation and Targeted Therapy published the research paper with the title of "Azvudine (FNC): a promisingclinical candidate for COVID-19 treatment", which reports the latest progress of Azvudine in the treatment of SARS-CoV-2 in detail. The report showed that Azvudine can treat the newly confirmed COVID-19 patients and obtain negative nucleic acid.
In mid-April, 2020, National Medical Products Administration approved the Phase III clinical trial of Azvudine for the treatment of COVID-19, and Phase III clinical research on the treatment of COVID-19 is carried out at home and abroad.
BRII-196 and BRII-198 are double-antibody cocktail combinations jointly developed by Brii Biosciences and Tsinghua University. Both antibodies originate from rehabilitation patients of COVID-19 and can bind different antigenic epitopes in RBD respectively. At present, the experimental data of chimeric virus in vitro showed that BRII-196 combined with BRII-198 could keep neutralizing activity against major SARS-CoV-2 variants such as Alpha, Beta, Gamma, Epsilon, Delta and Lamda.
In August of the year, BRII-196 and BRII-198 participated in overseas clinical Phase III clinical ACTIV-2, which is aimed at investigating the therapeutic effects of COVID-19 in early and late stage. The mid-term analysis of clinical trials showed that this cocktail therapy could effectively reduce the hospitalization and death risks of outpatients in COVID-19 by 78%. The result is positive. At the same time, another Phase II clinical trial has been carried out in China, aiming at investigating the therapeutic effect for mild and severe diseases, which is expected to be completed this year.
SCTA01 is an RBD neutralizing antibody independently developed by SinoCellTech, which can reduce virus entry into cells and pneumonia symptoms by binding RBD. At the same time, with the improvement of Fc segment, it reduces the adverse reactions of treatment.
In September last year, SCTA01 completed Phase I safety evaluation for healthy people in China. Up to now, SCTA01 has been approved to carry out international multicenter Phase II and Phase III clinical research on severe, mild/moderate and critically ill COVID-19 patients in the United States, Brazil, Mexico, Colombia, Peru, the Philippines and other countries. Clinical enrollment is underway and is expected to be completed from the end of 2021 to the first half of 2022.
Xiaoyaowan, a pharmaceutical industry practitioner, a word carrier in the We-media era focusing on changes of the pharma industry.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


