PharmaSources/YefenghongAugust 11, 2021
Tag: Hemophilia , Coagulation Factor VIII , Shenzhou Cell
On July 23, according to latest announcement in the official website of the National Medical Products Administration (NMPA), the recombinant coagulation factor VIII (SCT800) for injection developed by Shenzhou Cell Biotechnology (hereinafter referred to as Shenzhou Cell) has been officially approved. According to public information, this is the first Chinese-made recombinant coagulation factor VIII product. The indications of this approved product are as follows. It is suitable for controlling and preventing bleeding of teenagers and adults with hemophilia A (congenital coagulation factor VIII deficiency).
Hemophilia is a hereditary lifelong disease and its characteristic is blood coagulation cascade is damaged. Most patients are born with the lack of coagulation factors, so the normal touch will make them bleed. The critical patients will also have "spontaneous" bleeding even there is no obvious trauma. Hemophilia is divided into three types: A, B and C with the lack of coagulation factors VIII, IX and IX respectively.
The incidence of patients with hemophilia A is 1/10000, that of hemophilia B is 1/35000, and that of hemophilia C is rare. Almost all patients with hemophilia are male. Generally, women usually do not develop, but they can carry pathogenic genes, which leads to a 50% incidence rate for male offspring. Hemophilia A and B may be atavism, and the most common hemophilia A is caused by coagulation factor VIII deficiency or functional defect.
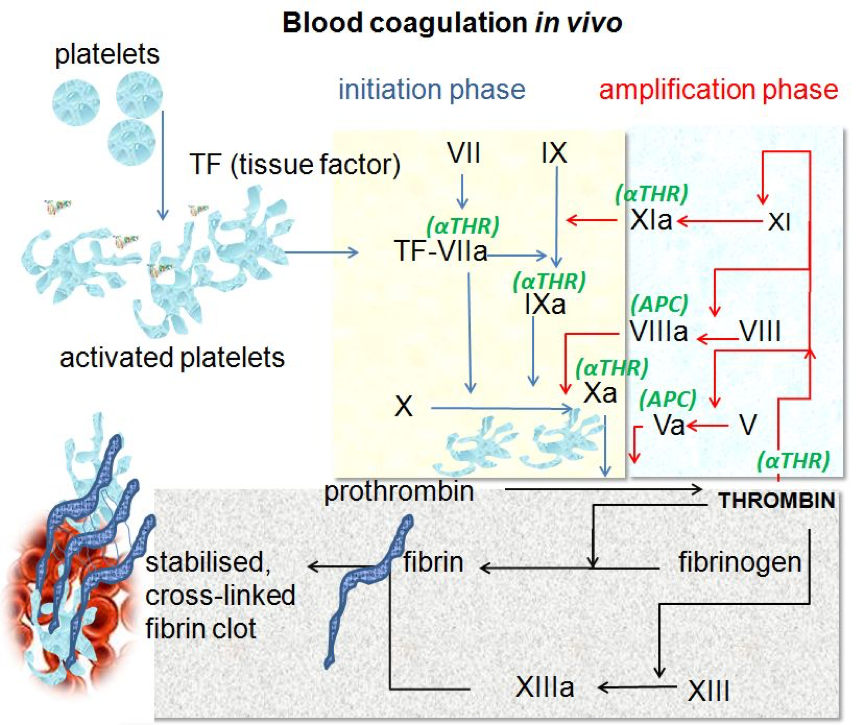
Blood coagulation cascade signaling pathway (source: References 1)
Although hemophilia is a rare disease, it does not represent that it is an incurable disease. It can reduce bleeding or stop bleeding by exogenously supplementing human-derived or re-giving coagulation factors.
At present, the manufacturers of coagulation factor VIII on the market in China are mainly Shanghai Raas, Hualan Biological Engineering, Inc., Green Cross, Taibang Biologic, Shanghai Xinxing Medicine Co., Ltd., etc., and all their products are the first generation of blood-derived coagulation factor VIII. While the imported products including Baxter's ADVATE, Bayer's Kogenate FS, Pfizer's Xyntha, etc. are mainly the recombinant coagulation factor VIII (second generation). In December 2020, the third generation of recombinant coagulation factor VIII for injection of Novo Nordisk was approved in China. If you are looking for bio pharmaceutical products, then Pharmasources would be your best choice.
The approved SCT800 is the third generation of recombinant coagulation factor VIII product developed by Shenzhou Cell, without albumin in its process and preparation. It is also the first recombinant coagulation factor VIII product in China. New drug application of this product was submitted in November 2019, and was then included in the priority review. According to the information on the official website of Shenzhou Cell, the Phase III on-demand treatment study of SCT800 for patients with hemophilia A and over 12 years old has completed, and Phase III preventive treatment study for patients with hemophilia A and over 12 years old is undergoing. At the same time, the Phase III preventive treatment study for patients with hemophilia A and under 12 years old has also been initiated.
In China, most coagulation factor VIII is extracted from human plasma. However, the productivity of extracting factor VIII from plasma is very limited because of the shortage of human plasma. At the same time, due to the poor thermal stability and low yield of coagulation factor VIII protein, as well as the high technical threshold and difficulty in the production of recombinant coagulation factor VIII protein, there is no domestic recombinant coagulation factor VIII protein drug that has been approved for marketing in China. While, the recombinant coagulation factor VIII developed by Shenzhou Cell is the first one in China.
There is a large population of hemophilia patients in China. According to the data of Consensus of Chinese Expert on the Diagnosis and Treatment of Hemophilia (Version 2017), the prevalence rate of hemophilia in China is 0.027‰, ranking first in the number of patients with rare disease in China. According to the statistical analysis of Frost & Sullivan, by the end of 2018, the number of hemophilia patients in China is close to 140,000, with a compound growth rate of 0.6%, of which hemophilia patients with hemophilia A account for about 85%. It is predicted that from 2019 to 2023, the number of hemophilia patients will continue to increase to 143,100 at an annual compound growth rate of 0.5%. From 2023 to 2030, the compound growth rate of patients will slow down. By the end of 2030, the number of patients will increase to 145,800 at an annual compound growth rate of 0.2%.
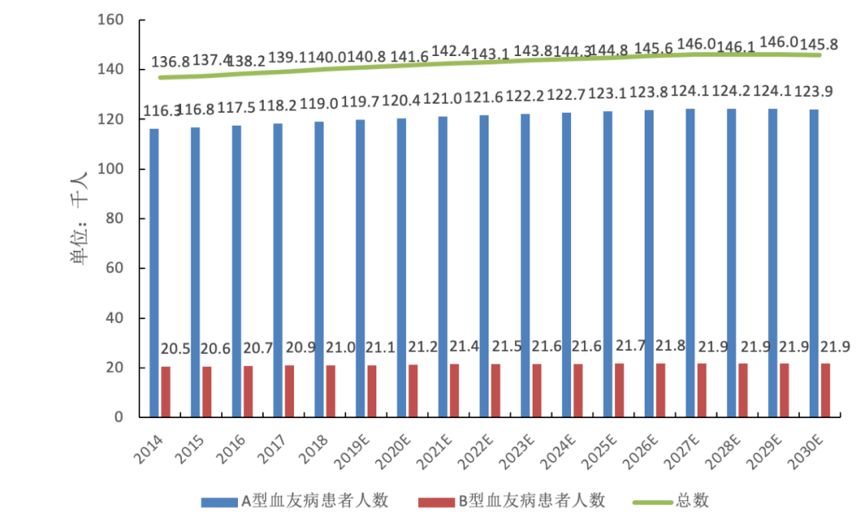
Overview of the number of hemophilia patients in China (Source: Frost & Sullivan Analysis)
Coagulation factor replacement therapy is the absolute mainstream of hemophilia therapy in the market at present, accounting for more than 99.9% of the total volume. However, in the next decade, more non-coagulation factor therapies and gene therapies will be approved to provide more treatment options for hemophilia patients.
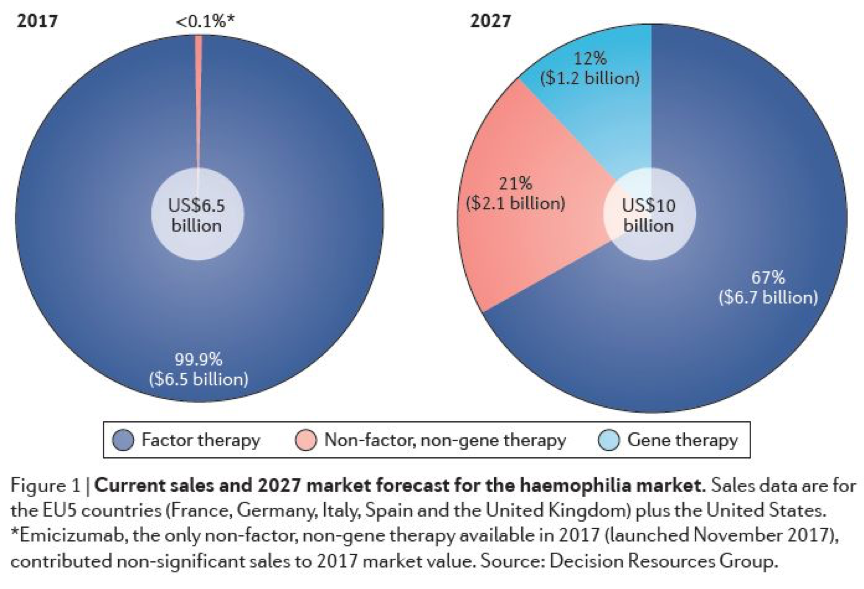
Market Share of Hemophilia Therapy in 2017 and Estimated Market Share in 2027 (Source: Reference 2)
Shenzhou Cell began to study recombinant coagulation factor VIII protein in 2008. On May 15, they submitted the first round of listing application to CDE. With the listing of recombinant coagulation factor VIII (SCT800), it can meet the drug need in China, as well as that in other countries around the world, greatly increasing the drug consumption of patients and reducing their economic burden.
Of course, a major question that Shenzhou Cell needs to think about is how to make its product outstanding among so many drugs after entering into the market.
1. https://creativecommons.org/licenses/by-sa/3.0;
2. Brown and Green, (2018). The haemophilia drug market. Nature Reviews Drug Discovery, https://doi.org/10.1038/nrd.2018.54.
Ye Fenghong, a medical editor specializing in oncology at a healthcare internet company, has conducted in-depth research on the pathogenesis and clinical treatment of lung cancer and breast cancer. She has previously been involved in the design and synthesis of anti-tumor drugs and has some experience in computer-aided drug design. She is currently devoted to introducing cutting-edge cancer treatment drugs to a wide range of readers, aiming to help more people avoid cancer pain and embrace good health.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


