Neeta RatanghayraAugust 06, 2021
Tag: BsAbs , Catumaxomab , Blinatumomab
Bispecific antibodies (BsAbs) are artificially designed antibodies with dual binding sites. The binding sites may be directed at two different antigens or two different epitopes on the same antigen. The dual binding ability makes bsAbs an attractive therapeutic option for a range of conditions including cancer, autoimmune diseases, and infectious diseases.
This article covers the basics of bsAbs and gives an overview of the bsAbs landscape in China.
Bispecific antibodies, with their novel functionalities, are seen as the next generation of cancer biotherapeutics. With bsAbs, it’s possible to combine more than one target which can ultimately help improve the efficacy and safety of biological pharma products with single targets. BsAbs can also help combat drug resistance commonly observed with a single target antibody.
BsAbs are available in multiple formats ranging from small protein fragments to large immunoglobulin G (IgG)-like molecules and its dual binding ability enables a flexible functioning pathway.
The specificity of bsAbs can be leveraged to redirect T cells to cancer cells or block two different signal transduction pathways simultaneously. BsAbs can also be used for simultaneous targeting of multiple disease mediators and targeted delivery of payloads into tumor cells. Several bsAbs are designed to act as connectors of immune cells and work by connecting immune cells to cancer cells, thereby allowing immune cells to exert their killing action.
Most BsAbs are designed to target immune cells CD3, CD16, and CD47, while some are designed to enable dual immunomodulation and targets dual-targeted immune checkpoints pathways such as PD-1, PD-L1, LAG-3, and TIM-3.
In addition to targeting immune cells, BsAbs can target dual tumor targets such as HER2, EGFR, DLL1and block dual signaling pathways. They can also target inflammatory factors in the tumor microenvironment and reduce inflammation and cytokine release syndrome.
Catumaxomab, a murine anti-EpCAM × anti-CD3 bsAb was the first bsAb drug approved in the world. Catumaxomab (Removab®) was approved in the EU for the intraperitoneal treatment of malignant ascites in patients with EpCAM-positive epithelial tumors. However, catumaxomab was voluntarily withdrawn by its manufacturer in 2017 due to commercial reasons.
Chinese biotech Lintonpharm is reinitiating the clinical development of catumaxomab. Lintonpharm has received authorization from China’s National Medical Products Administration (NMPA) to conduct a global phase III trial of catumaxomab in advanced gastric cancer. The NMPA has also authorized Lintonpharm to proceed with a Phase 1/2 clinical trial evaluating the safety and efficacy of catumaxomab in patients with non-muscle-invasive bladder cancer (NMIBC) whose tumors have recurred due to Bacillus Calmette-Guerin (BCG) vaccine failure.
Blinatumomab (Blincyto®) developed by Amgen, is the second bsAb to be introduced in the market. Blinatumomab is an anti-CD19 × anti-CD3 antibody indicated for the treatment of acute lymphoblastic leukemia.
The third bsAb to be approved is Roche’s Emicizumab (Hemlibra®). Emicizumab is an anti-FIXa × anti-FX antibody that mimics activated factor FVIII (FVIIIa), a clotting protein reduced in patients with hemophilia A.
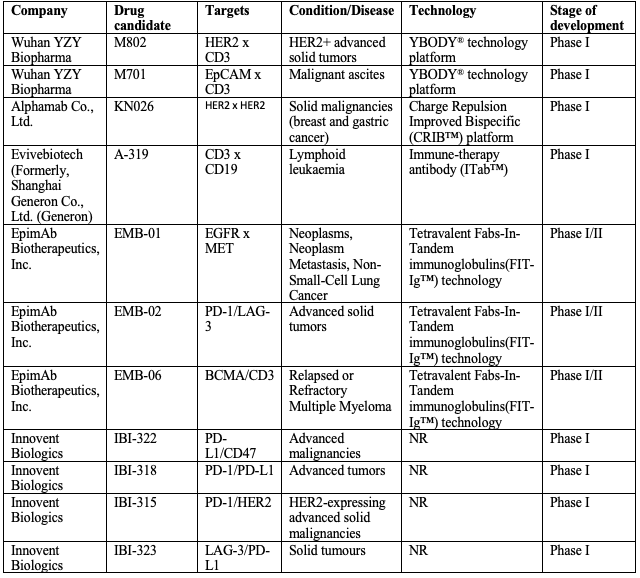
The attractive features of bsAb have led to significant efforts towards their development as the next generation of biotherapeutics, especially in cancer. In addition to the two bsAbs currently approved, around 110 bsAbs are currently at various phases of clinical development. Of this, 21 clinical trials are being carried out in China. Most of the bsAbs being evaluated in China target solid tumors while few target hematological tumors including lymphoma and cancer-caused malignant ascites.
Several platforms for designing bsAb have been developed in China, of which many have been received patent from the US Patent Office.
Major bsAb platforms developed in China include
YBODY® (Wuhan YZY Biopharma)
CRIB™ (Alphamab)
ITab™ (Shanghai Generon)
FIT-Ig™ (EpimAb Biotherapeutics)
WuXiBody™ (WuXi Biologics)
SMAB™ (Genscript Biotech)
Though poised to be the next-generation biologics, development and manufacturing challenges hamper the wider use and clinical acceptance of bsAb.
Some of the key technical challenges while developing bsAb include
The requirement of high expression level and production yield makes it difficult to develop productive processes
Requirement of strategies to prevent toxicity and immunogenicity caused by novel epitopes
Meeting thresholds for activating multiple molecular pathways
Ensuring the product quantity, quality, and stability during manufacturing
Currently, there are only two commercially available bsAbs, but the way the approved bsAbs have revolutionized the treatment landscape reflects its great market potential. As per recent reports, the estimated market opportunity for bsAbs is set to reach $8 billion by 2025.
Also, the growing number of bsAbs candidates authorized for clinical trials by NMPA and the continuous financial investments reflect the advancing innovation and competitiveness of Chinese biotech companies.
1. Zhang J, Yi J, Zhou P. Development of bispecific antibodies in China: overview and prospects. Antib Ther. 2020;3(2):126-145.
2. Labrijn AF, Janmaat ML, Reichert JM, Parren PWHI. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019;18(8):585-608.
3. Global Bispecific Antibody Market, Drug Sales & Clinical Pipeline Insight 2025. Available at: https://www.researchandmarkets.com/research/xhhw54/global_bispecific?w=5. Accessed on: 29 Jun 2021.
Neeta Ratanghayra is a freelance medical writer, who creates quality medical content for Pharma and healthcare industries. A Master’s degree in Pharmacy and a strong passion for writing made her venture into the world of medical writing. She believes that effective content forms the media through which innovations and developments in pharma/healthcare can be communicated to the world.
This article is first published on
Pharma Sources Insight August 2021



Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


