PharmaSourcesJune 22, 2021
Tag: levodopa , API Powder , decarboxylase
Levodopa is a precursor of dopamine that is commonly used in the treatment and management of Parkinson’s disease because of its ability to cross the blood brain barrier. It is usually formulated with a dopa decarboxylase inhibitor such as carbidopa. Brand names of the combination products include Sinemet, Sinemet CR, and Parcopa.
Levodopa is available in a couple of different forms and specifications including levodopa API powder and carbidopa-levodopa combination.
Raw material of levodopa API powder (CAS 59-92-7) is in the form of a white to almost white crystalline powder. The specification from Chinese manufacturers Shandong Lukang Pharmaceutical Co., Ltd., Chengdu Okay Pharmaceutical Co., Ltd., Shandong Xinhua Pharmaceutical Co., Ltd., Zhejiang Huahai Pharmaceutical Co., Ltd., Guangxi Changzhou Natural Pharmaceutical Co., Ltd., and Zhejiang Wild Wind Pharmaceutical Co., Ltd. is 99% min.
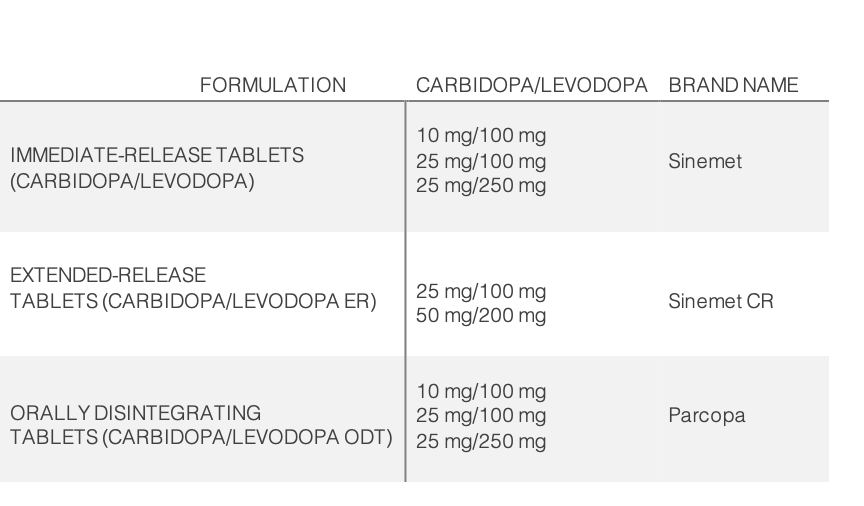
Levodopa is available in combination with carbidopa, a dopa decarboxylase inhibitor. This combination product is available in immediate-release tablets, extended-release tablets, and orally disintegrating tablets as detailed in the table below:
Levodopa is a precursor to dopamine used in the treatment of Parkinson’s disease because of its ability to cross the blood brain barrier. Ethinyl estradiol is a hormone medication used for birth control and other hormonal conditions.
Levodopa is a precursor to dopamine used in the treatment of Parkinson’s disease because of its ability to cross the blood brain barrier. Norgestimate is a hormone medication used for birth control and other hormonal conditions.
Levodopa is the precursor to dopamine. It crosses the blood brain barrier to form dopamine through decarboxylation. This form of dopamine functions in a way that endogenous dopamine cannot because of a reduction in natural concentrations and stimulates dopaminergic receptors (DrugBank).
The manufacturing process of levodopa API is conducted under proper quality control systems in compliance with the regulations of international Good Manufacturing Practice (GMP) to produce safe and high-quality active pharmaceutical ingredients.
Levodopa comes from different types of beans such as vanilla bean. The manufacturing process of levodopa involves “preparation from L-tyrosine then production: glycine + acetic anhydride + vanillin (amide formation/carbonyl condensation/chiral catalytic hydrogenation/hydrolysis). Catechol + pyruvic acid + ammonia (microbial conversion)” (PubChem Compound Summary for CID 6047, Levodopa).
Impurities are then extracted from the active ingredient. Levodopa undergoes testing and purification in accordance with GMP and quality standards until it achieves 99% min purity specification.
Levodopa is used to treat and manage Parkinson’s disease. L-DOPA may also be beneficial for bodybuilding by causing an increase in testosterone; however, this has not yet been studied in humans.
Levodopa, combined with a dopa decarboxylase inhibitor such as carbidopa, is usually started at a low dose and slowly titrated up for each patient based on their therapeutic response (MedScape). According to MedScape, many patients have a good therapeutic response while taking 300-600 mg per day (in divided doses 3 or 4 times daily) of levodopa. The dosage should be individualized, monitored, and appropriately adjusted for each patient based on their therapeutic response.
Side effects may include dizziness, diarrhea, dry mouth, a change in sense of taste, headache, loss of appetite, weakness, constipation, and nervousness.
You could find professional and trustworthy API pharmaceutical wholesalers on Pharmasources.
Chinese manufacturers, Shandong Lukang Pharmaceutical Co., Ltd., Chengdu Okay Pharmaceutical Co., Ltd., Shandong Xinhua Pharmaceutical Co., Ltd., Zhejiang Huahai Pharmaceutical Co., Ltd., Guangxi Changzhou Natural Pharmaceutical Co., Ltd., and Zhejiang Wild Wind Pharmaceutical Co., Ltd.
manufacture raw material of levodopa API powder 99% in bulk. If you need a price quote, certificate of analysis (COA), or a detailed specification sheet, please visit these professional manufacturers on www.pharmasources.com.
Established in 1966 and listed in Shanghai Stock Exchange, Shandong Lukang Pharmaceutical Co., Ltd. is China’s top manufacturer of antibiotic products. With 45 years of pharmaceutical manufacturing experience, they specialize in the research, manufacturing, and sales of API, pharmaceutical intermediates, and finished products for human, vet, and agricultural use. Manufacturing safe and high-quality products in a safe environment while keeping their employees safe and healthy is their priority. They hold ISO 9001, ISO 14001, and ISO 18001 certifications. They also hold GMP certification and regularly undergo inspections by the State Food and Drug Administration.
For more information about Shandong Lukang Pharmaceutical Co., Ltd., visit www.pharmasources.com.
Established in 2004, Chengdu Okay Pharmaceutical Co., Ltd. is technologically advanced, specializing in plant extractions and purification and synthesis. They also manufacture raw materials of health products, drugs, cosmetics, and functional foods. They hold GMP standards.
For more information about Chengdu Okay Pharmaceutical Co., Ltd., visit www.pharmasources.com.
Established in 1943, Shandong Xinhua Pharmaceutical Co., Ltd. is China’s largest chemical synthetic pharmaceutical manufacturing and exporting base. They specialize in chemical production for manufacturing APIs. Nine products have been approved by COS, and nine products have been approved by the FDA.
For more information about Shandong Xinhua Pharmaceutical Co., Ltd., visit www.pharmasources.com.
Established in 1989, Zhejiang Huahai Pharmaceutical Co., Ltd is now listed in Shanghai Stock Exchange. They are modern and large-scaled, specializing in APIs and intermediates. They are titled National Key Hi-tech Enterprise, National Pilot Enterprise of Innovation, and China’s top 500 private enterprises while holding a State Certified Enterprise Technology Center.
They are the world’s largest supplier of pril products, with captopril and enalapril manufacturing ranking first in the world. Known as the “Pril Specialists,” they are also the world’s only manufacturer who can produce captopril, enalapril, and lisinopril simultaneously. They hold international GMP and USA FDA Approval.
For more information about Zhejiang Huahai Pharmaceutical Co., Ltd., visit www.pharmasources.com.
Established in 1999, Guangxi Changzhou Natural Pharmaceutical Co., Ltd. is technologically advanced, specializing in the research and manufacturing of natural products. They are one of the largest research and manufacturing companies in Asia, exporting their products to Europe, United States, and Southeast Asia.
For more information about Guangxi Changzhou Natural Pharmaceutical Co., Ltd., visit www.pharmasources.com.
Established in 1993, Zhejiang Wild Wind Pharmaceutical Co., Ltd. specializes in the manufacturing of L-methyldopa, Carbidopa, and more. They also manufacture raw medicine, polymer material, and intermediates. They hold the titles of China’s Best Enterprise Image AAA Grade, The Credited Enterprise AAA Grade, The Largest Industrial Enterprise in Zhejiang Province, and Zhejiang Provincial Excellent Scientific and Technological Advanced Enterprise. They are currently striving to obtain ISO 9001 and ISO 14001.
For more information about Zhejiang Wild Wind Pharmaceutical Co., Ltd., visit www.pharmasources.com.
Pharmasources.com is a professional platform, committed to providing information about the highest-quality pharmaceutical products and suppliers to their global customers. They deliver services to 900,000+ active members across 211 countries and regions, while featuring more than 1,600,000 products in 30 major categories that span the entire pharma supply chain. Their mission is “to connect global buyers with quality suppliers and facilitate easy trade of the pharmaceutical industry” (PharmaSources).
According to MarketWatch, the top levodopa suppliers are Xi’an Lyphar Biotech, Hindustan Herbals, Xi’an ZB Biotech, Xi’an Arisun ChemPharm, and Hunan Nutramax.
According to Markets and Markets, in 2016, the global Parkinson’s disease drugs market was USD $3.99 billion. It is expected to grow at a CAGR of 6.1% through 2022, reaching USD $5.69 billion.
1. Carbidopa/Levodopa ER. GoodRx. https://www.goodrx.com/carbidopa-levodopa-er. Accessed June 12, 2021.
2. Company Profile of Chengdu Okay Pharmaceutical Co., Ltd. PharmaSources. https://www.phar
masources.com/company-profile/chengdu-okay-pharmaceutical-co-ltd-userId-551.html. Accessed June 13, 2021.
3. Company Profile of Guangxi Changzhou Natural Pharmaceutical Co., Ltd. PharmaSources. https://www.pharmasources.com/company-profile/guangxi-changzhou-natural-pharmaceutical-userId-21558.html. Accessed June 13, 2021.
4. Company Profile of Shandong Lukang Pharmaceutical Co. PharmaSources. https://www.pharma
sources.com/company-profile/shandong-lukang-pharmaceutical-co-ltd-userId-968.html. Accessed June 13, 2021.
5. Company Profile of Shandong Xinhua Pharmaceutical Co. PharmaSources. https://www,pharmas
ources.com/company-profile/shandong-xinhua-pharmaceutical-co-ltd-userId-160.html. Accessed June 13, 2021.
6. Company Profile of Zhejiang Huahai Pharmaceutical Co. PharmaSources. https://www.pharmaso
urces.com/company-profile/zhejiang-huahai-pharmaceutical-co-ltd-userId-16662.html. Accessed June 13, 2021.
7. Company Profile of Zhejiang Wild Wind Pharmaceutical Co. PharmaSources. https://www.pharm
asources.com/company-profile/zhejiang-wild-wind-pharmaceutical-co-ltd-userId-56711.html. Accessed June 13, 2021.
8. Global Levodopa Market Size 2021-2026 With Top Countries Data: Industry Overview by Size, Share, Future Growth, Development, Revenue, Top Key Players Analysis and Growth Factors | With Covid 19 Analysis. MarketWatch. https://www.marketwatch.com/press-release/global-levodopa-market-size-2021-2026-with-top-countries-data-industry-overview-by-size-share-future-growth-development-revenue-top-key-players-analysis-and-growth-factors-with-covid-19-analysis-2021-03-22. Published March 22, 2021. Accessed June 13, 2021.
9. Hauser, Robert MD. What is the recommended dosage for levodopa and a dopa decarboxylase inhibitor in the management of Parkinson disease (PD)? MedScape. https://www.medscape.com/answers/1831191-9932/what-is-the-recommended-dosage-for-levodopa-and-a-dopa-decarboxylase-inhibitor-in-the-management-of-parkinson-disease-pd. Updated August 29, 2019. Accessed June 13, 2021.
10. L-DOPA. MRSUPPLEMENT. https://www.mrsupplement.com/au/ldopa#:~:text=L%2DDOPA%20
Benefits%20for%20Bodybuilding,to%20an%20increase%20in%22testosterone.&text=However%2C%20human%20growth%20hormone%20has,results%20at%20dosages%20over%201000mg. Accessed June 13, 2021.
11. Levodopa. DrugBank. https://go.drugbank.com/drugs/DB01235. Accessed June 12, 2021.
12. Levodopa and Carbidopa. MedlinePlus. https://medlineplus.gov/druginfo/meds/a601068.html. Last revised June 15, 2018. Accessed June 12, 2021.
13. National Center for Biotechnology Information. "PubChem Compound Summary for CID 6047, Levodopa" PubChem, https://pubchem.ncbi.nlm.nih.gov/compound/Levodopa. Accessed 12 June 2021.
14. Parcopa Carbidopa/Levodopa. GoodRx. https://www.goodrx.com/parcopa. Accessed June 13, 2021.
15. Parkinson’s Disease Treatment Market by Drug Class (Carbidopa/Levodopa, Dopamine Receptor Agonists, MAO-Inhibitors), Distribution Channel (Hospital, Online, Retail Pharmacies), Patient Care Setting (Hospitals, Clinics) – Global Forecast to 2022. MarketsandMarkets. https://www.marketsandmarkets.com/Market-Reports/parkinson-disease-treatment-market-47265247.html#:~:text=%5B113%20Pages%20Report%5D%20The%20Parkinson's,highest%20CAGR%20during%20forecast%20period. Accessed June 12, 2021.
16. Sinemet Carbidopa/Levodopa. GoodRx. https://www.goodrx.com/sinemet. Accessed June 12, 2021.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


