Deepak HegdeJune 16, 2021
The process of taking a new drug from concept to clinic and eventually to commercialization involves several steps which traditionally occur in series. The average development timeline from discovery stage to commercialization today takes anywhere from 10 and 14 years with costs more than $1 billion.1,2 with a success rate of only one in 10000 discovery compounds reaching the market. Change from sequential to overlapping value chain to reduce cycle times would benefit the pharmaceutical industry tremendously. Increasingly, in-vivo studies are conducted in parallel. Failing faster and cheaper is becoming increasingly important for the pharmaceutical industry! This can be helped in no mean measure by application of rational formulation development.
Formulations span the whole life cycle of NCE spectrum from discovery to commercialization and beyond as shown in Fig 1.
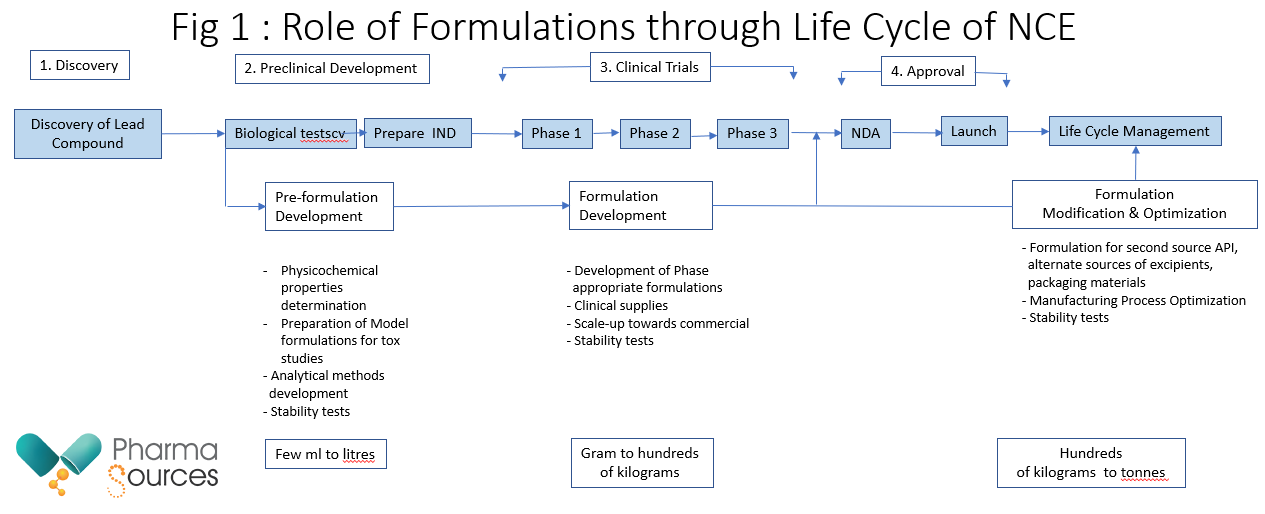
Pre-formulation studies are highly important to characterize new molecules and forms the basis for starting any formulation effort. It helps in understanding the critical properties of the NCE and its fault lines that need to be overcome by the formulation process. Among the key properties of the Active Pharmaceutical Ingredient (API) to be characterized include melting point, microscopy, solubility (pH 1.2, pH 7.4), Log P, pKa, permeability, chemical stability, particle size distribution, microscopy, density, solubility (pH range1–8, biorelevant dissolution media), Log P, wettability, flow properties, compression properties, excipients compatibility and hygroscopicity. As different polymorphic forms could co-exist for the same NCE, it is very important to identify a stable polymorphic form and make sure that the same form is used during the different stages of drug development, so that adequate exposure can be maintained.
Phase 1 involves API characterization including particle size, flow properties and other critical physical characteristics, development of prototype formulations, selection of excipients and definition of ratios versus API. Thus, the goal is to define a formulation.
Phase II involves scale-up to appropriate scale and optimization of both formulation and manufacturing process. This the goal is to finalize quantitative formulation including in-process tests and release specifications
Phase III involves scale up to pivotal batch scale, up to 10% of commercial batch size (or 100,000 units), finalization of the manufacturing process, use of validated analytical methods and manufacture of registration stability batches using the API with the final synthetic process and batch size for supporting market expiration date using the final shape, color, printing or embossing of tablets intended for commercial, development of packaging for both commercial product & physician’s samples, validating hold times in manufacturing, finalizing rework procedures and finalization of manufacturing process. Thus, the goal is ensuring readiness for NDA Filing.
NDA Filing/ Commercialization stage involves the manufacturing of validation batches, finalization of protocols to be filed in NDA and finalizing the timing of execution, Readiness for Pre-Approval Inspection (PAI) audit. Thus, the goal is successful validation, ensuring launch readiness and to pass PAI
During the process of formulation development, the objective is to develop a formulation and process that can consistently deliver the drug to the patients with appropriate quality attributes. A good formulation is one that is able to assimilate the variability seen with the API, excipients as well as the manufacturing process without compromising on the performance of the product throughout the shelf life. Moving along, the commercialization objective is to ensure that the manufacturing process is well understood and standardized, all possible sources of variability are well understood, characterized and explained, variability within API, excipients and manufacturing process is well managed, Product Quality Attributes (PQA’s) are well predicted through the design space, which is appropriately designed, and space maintained for improvement through commercial life of product.
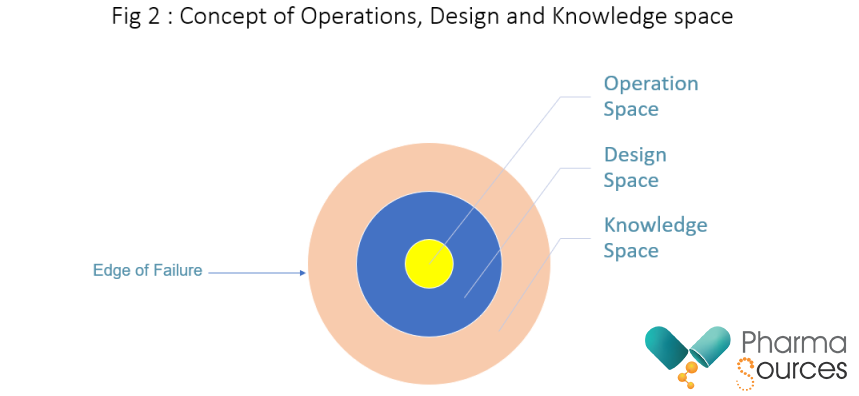
The concept of rational formulation development is very relevant and should guide the development of a product. Quality is not something that can only be tested at the end to ensure a good product. It is something that needs to be built into the product from the very beginning. During the development stage, Quality by Design (QBD)3, should be the guiding principle during the design of the formulation and should be used to evaluate and optimize manufacturing process and formulation. During the optimization of the formulation, and the manufacturing process, the product specification needs to be challenged by collection, evaluation, and trending of data. This ensures the building of database which becomes the repository for any corrective actions in the future for addressing process deviations, challenging product quality characteristics and for risk assessments and mitigation strategies. Building a database for a product helps in the full understanding of product and the process of manufacture, full characterization of API and excipients using statistical experiments, defining acceptable operating ranges for manufacturing process and demonstrating operability at full scale. Thus, during the process of formulation development, the concepts of operation space, design space 4 and knowledge space as shown in Figure 2, as should be fully exploited.
During the development of formulations, Design of Experiments (DoE) is an essential part of QBD, as it ensures a strong justification for the design space, helps in establishing optimum manufacturing parameters for robust and reproducible manufacturing, minimizing batch to batch manufacturing variations and provides support for in-process and release specifications for the product. It is often asked as to what the right time is for performing DOE studies during the process of formulation development. Typically, it is best to start DOE studies when the manufacturing process along with the manufacturing equipment train is established and strengths of the product are already defined. This would typically be at end of Phase IIB or early Phase III. Experimental designs should include screening studies using a design like Plackett Burman design. Historical data should be reviewed and as many relevant parameters as possible should be studied to identify critical parameters by performing trial runs at extremes. Designs should include full factorial design with center points, characterizing magnitude with effects, using center points to estimate variability, establishing interactions and using scientific justification of manufacturing parameters. Multiple studies should be carried out for unit operations (e.g., for tablet product- blending, granulation, compression).
While scaling up formulations, it is very important to make sure that the API characteristics are consistent from that from smaller lots as this can have a significant effect on the scaleup efforts. In terms of physical characteristics attention to be paid to the physical stability in terms of moisture sensitivity, changes in particle shape and particle size distribution which can cause flowability issues in scale-up batches. Among the chemical characteristics, a key attribute to check is consistency of polymorphs in scaled up batches of API as compared to earlier smaller scale ones.
Robustness is the ability of a process to demonstrate acceptable quality and performance, while tolerating variability in inputs. It is a function of formulation and process design. It is very important that control capability when processing at pilot versus manufacturing scale must be understood. Experimentation In manufacturing is limited as compared to research and development and the state of robustness can be determined via proactive process monitoring.
A key step towards commercialization is TT and the goal of TT activities is to transfer product and process knowledge. TT Is a valuable step in the developmental life cycle leading to successful commercial manufacturing. In TT, we take all the gathered knowledge and use it as the basis for the manufacturing control strategy and the approach to process qualification and on-going continuous improvement. We transition the product, process and analytical method knowledge between development and manufacturing sites. This is done to ensure that the variability of process and parameters are controlled and sufficient in the face of the rigors of a commercial production environment, to verify that the parameters established during development are still within the determined design space and are adjusted at scale-up.
During the process of TT, a diverse skilled and collaborative team is formed including development and receiving site personnel. They review process flow diagram for key inputs and outputs that could impact Quality Risk Management (QRM). Uni/ multi variant experiments should be completed to study relationships and gain information on potential sources of variability with a need to know where quality could be impacted. During this process, the measurement capability (i.e., repeatability, precision) is well understood and Critical Process Parameters (CPPs), Critical Quality Attributes (CQAs) and other important parameters are identified. Design space should be defined and understood consisting of a set of input ranges (CPPs) that provide high probability that CQAs will meet specification. Finally, a control strategy needs to be in place to assure focus on critical points
Using sound scientific principles-based approach,QBD, to formulation development supports the development of a robust/safe product. Approaches to development require a certain degree of rigor to enable a robust technology transfer commercialization. Complexities in scale-up should not be underestimated. Successful formulation development which can be transferred to commercial is a collaborative effort with Research and Development, Manufacturing Technical Operations, Quality, Manufacturing etc. that is needed to assure a successful technology transfer and a robust final manufactured product.
1. Sullivan T. A Tough Road: Cost to Develop One New Drug Is $2.6 Billion; Approval Rate for Drugs Entering Clinical Development is Less Than 12% [Internet]. Policy & Medicine. 2018 [cited 1 February 2019]. Available from: https://www.policymed.com/2014/12/a-tough-road-costto-develop-one-new-drug-is-26-billion-approval-rate-for-drugsentering-clinical-de.html.
2. Sertkaya A; Wong H. H.; Jessup, A; Beleche, T (2016). Key cost drivers of pharmaceutical clinical trials in the United States. Clinical Trials. 13 (2): 117–26. doi:10.1177/1740774515625964. PMID 26908540.
3. Guidelines Q8, Q9, Q10 and Q11 from the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH).
4. Guideline Q8 (R2) on pharmaceutical development from the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH).

Deepak Hegde, Ph.D., M.F.M, is an industrial pharmacist by training. He has a been involved in development and commercialization of both innovative and generic drugs from a very early phase of development to technical transfers for commercial manufacturing sites, for the past 25 years. During his career, he has worked Rhone Poulenc, Novartis (Sandoz), USV Ltd., WuXi AppTec and GSK. He is currently working with EOC Pharma. as Chief Technology officer.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


