PharmaSources/Zhulikou431June 03, 2021
Tag: Complex Generic Drugs , FDA , active ingredients
Since the promulgation of the Hatch-Waxman Act in 1984, in order to promote drug competition and improve public drug availability, FDA has formulated a number of policies aimed at accelerating the marketing of generic drugs. But with the development and approval of newer and more unique products, the complexity of drugs is also increasing. Many drugs have developed from simple small molecules to products with complex active ingredients, dosage forms, formulations or routes of administration, as well as products as a combination of drugs and machinery. Complex drugs have brought new challenges.

According to the definition of FDA's GDUFAII commitment letter, complex products generally include the following situations:
1.Products with complex active ingredients (e.g. peptides, macromolecular compounds, complex mixtures of APIs, ingredients from natural sources); Complex formula (such as liposome, colloid); Complex routes of Administration (for example, topical agents, such as Department of dermatology products, complex ophthalmic products, ear preparations formulated with suspension, emulsion or gel), or complex dosage forms (such as transdermal drugs, metered dose inhalers, sustained-release injections).
2. Complex drug - device combination products (e.g. automatic syringe, metered dose inhaler)
Other complexities or uncertainties associated with approval pathways or possible alternatives will benefit from products with early scientific involvement.
For these complex generic drugs, since the complex formulations and active ingredients, it is difficult to "copy" them (such as their formulations or drug delivery systems) by using traditional biological methods. Therefore, the number of the product is small, resulting in less market competition for these products. On the other hand, these products were not foreseen in the generic drug approval route determined at the beginning of the hatch Waxman act, and special evaluation of product attributes was needed during the review. In recent years, FDA has also paid special attention to complex generic drugs. From 2014 to 2020, FDA has basically listed "equivalence of complex products" as the focus of scientific research in GDUFA supervision, striving to improve and promote the process of R & D, application and approval of complex generic drugs, and improve the efficiency of application and listing of complex generic drugs.
On the one hand, FDA has issued a series of product specific guidelines (PSG), which describe FDA's current ideas and expectations on how to develop generic drugs equivalent to specific reference drugs. This will help the generic drug industry to determine the most appropriate method and evidence to support the approval of specific generic drugs. FDA has a database of PSG guidelines, which is updated regularly.
In addition, in order to pay special attention to complex generic drugs, FDA has opened a new webpage for PSG of complex generic drugs “Upcoming Product - Specific Guidances for Complex Generic Drug Product Development”. When FDA releases PSG batches every quarter, the webpage will be updated. Each update will delete the published PSG and add any new PSG under development or revision. This advance notice will help generic companies and wholesale medical supply companies plan or project their development of complex generic products. Generic drug applicants can also specifically track the new and revised guidelines for complex products planned by FDA, so as to avoid the lack of preventive measures due to the revised guidelines in the development or application review.
On the other hand, FDA has established pre-ANDA program according to the generic drug user fee amendment in 2017 (GDUFA II), which aims to clarify the regulatory expectations of applicants through written communication and meetings at the early stage of drug development, assist applicants to submit complete applications, improve the efficiency of the review process, and reduce the approval rounds required for the approval of complex generic drugs. There are three types of Pre-ANDA meetings: Product development meeting, Pre-submission meeting and Mid-review cycle meeting. The program pays special attention to complex generic drugs. On November 25, 2020, FDA released the final draft guide of "formal meeting between ANDA applicants and FDA for complex products under GDUFA ", which outlines when to hold and apply, and opening procedures of the three meeting types, which are product development, pre-submission and mid-term review meetings. The convening and full communication of these meetings will build a bridge of information transmission between the industry and FDA.
On August 1, 2020, the FDA awarded the University of Maryland and the University of Michigan a five-year grant (August 1, 2020 to July 31, 2025) to establish the Center for Research on Complex Generics (CRCG). This funding is aimed at strengthening research cooperation with the generic industry to further realize OGD's mission of increasing access to safe, effective, high-quality and potentially low-cost generic drugs. CRCG will achieve this goal through collaborative research, training, workshops, laboratory projects to meet development needs, complex generic Pharma program, and resource exchange among FDA, generic industry, and stakeholders.
On May 19, 2021, FDA issued the second batch of 21 product specific guidelines (PSG), including guidelines of fluticasone furoate, a complex product; Umenium bromide; Winant Lo inhaled powder (RLD:TRELEGY ELLIPTA), midazolam nasal spray (RLD: NAYZILAM), methoxypropanamide nasal spray (RLD: GIMOTI) and intravenous meloxicam solution (RLD: ANJESO). We can see from FDA's PSG guidelines database that up to now, FDA has published 1896 product specific guidelines.
Now let's try to understand the specific situation of complex generic drugs through several sets of data and cases:
In early 2021, at the webinar "Complex Generics and Prescription Patterns" held by The Hill and sponsored by AAM, the participants mentioned the approval of complex generics outside the U.S. market. See the following table for details:
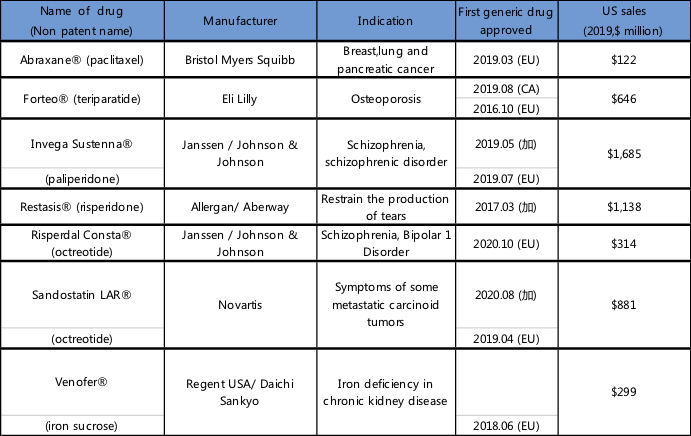
Source: Shilin
In addition, we can see from the annual reports of generic drugs released by the FDA Office of generic drugs (OGD) in recent years that in 2018, FDA approved or temporarily approved 1021 simplified drug applications (ANDAs), of which 14% were complex generic drugs; In 2019, a total of 1014 generic drugs were finally approved and initially approved, of which 110 were complex generic drugs, accounting for 10.8%.
Some of the complex generic drugs approved by OGD in fy2021 are as follows:
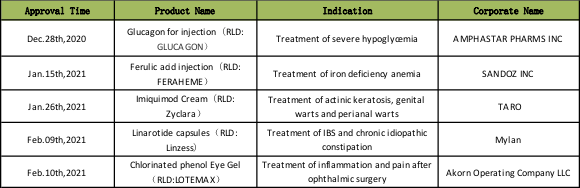
Although the profits left by the US generic market to the latecomers are not huge, the current situation is not so for the complex generic enterprises. Because of the technical barriers and regulatory problems of complex generic drugs, if a generic drug company is actively committed to these areas with insufficient competition, it can get better feedbacks through efforts. Compared with innovative drugs, the failure probability of complex generic drugs is much lower, which is really a good choice for some enterprises. It is hoped that Chinese enterprises can seize the opportunity to actively expand in this field and strive to continuously enhance the voice of the industry.
FDA Website
Review and Policy Focus Summarized at FDA 2021 Generic Forum
“Alex Brill, Potential Savings from Accelerating US Approval of Complex Generics”
Zhulikou431, as a senior engineer, PDA member, ISPE member, ECA member, PQRI member, senior aseptic GMP expert, has deep knowledge in aseptic process development and verification, drug development and registration, CTD document writing and review, regulatory audit, international certification, international registration , quality system construction and maintenance, as well as sterile inspection, environmental monitoring and other fields. In recent years, he has focused on the analysis of trends in the macro pharmaceutical field and the risk management of pharmaceutical enterprise mergers and acquisitions projects.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


