Noor SalehMay 28, 2021
Tag: Industry 4.0 , Digital Transformation , big data
Have you ever imagined as an expert in drugs manufacturing that Industry 4.0 can be applied in pharma world? Believe it or not! The future now is for the autonomous, or as called SMART factories and operations.
Industry 4.0 refers to the Fourth Industrial Revolution. Indeed, the industrial revolution has gone into four stages as illustrated in the following Figure No. 01:
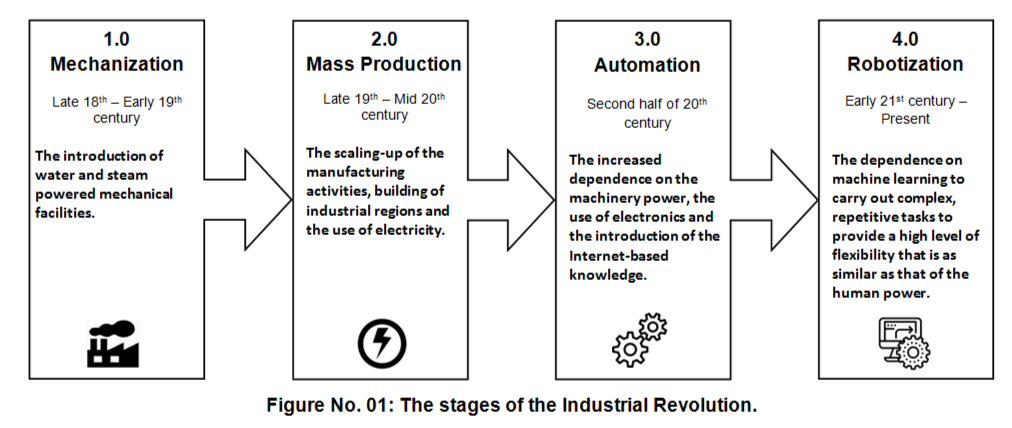
Digital transformation literally means: the application of technology to transform any process from being manual to a digital or an automated one. In other words, Digital Transformation is as same as the concept of Business Process Automation.
On the other hand, Robotization means: the introduction of robots to carry out the industrial tasks. So, this word refers to the automation of a process by the use of a robotic device.
Eventually, while these terms are not usually used interchangeably, in both cases, the applicant is interested in the creation of an automated (i.e. digital) business process in order to increase the quality of the output while decreasing the cost of poor quality.
1. Additive Manufacturing AM: this term refers to the use of a set of technologies to build a three-dimensional 3D model using a 3D software in a fast and customized way. There are many applications of this trend, including but not limited to: the printing of a Polypill, which helps tailor the medications for each patient based on his needs. A Polypill containing specific amounts of different Active Pharmaceutical Ingredients APIs, depending on the underlying medical conditions, can be printed in the hospital setting and given to a specific patient. The use of Polypill is expected to have many advantages, such as: increasing the adherence of elderly patients to their medications (i.e. eliminates the need to administer different types of pills during the day), on-demand printing of any drug in case of emergencies, and since the Polypill will be taken soon after being 3D printed, this might help to reduce the need of conducting a long-term stability study to evaluate the API shelf-life.
2. Augmented Reality AR: augmented reality activities help to establish 3D graphics in a real-world environment, where models or prototypes of products and devices can be examined in their real size, magnified or minimized. As an example, a surgeon can utilize this technology to build a 3D kidney model before doing a partial nephroectomy (i.e. surgical removal of a kidney) to help him take the proper surgical decision. Also, a doctor can build a 3D model for a specific organ to virtually study the progression of a certain disease, to evaluate the mechanism of action of a potential therapeutic entity, or to educate the patient about his medical condition. Another interesting application is the process simulation and modeling, by which, a mathematical model can used to build a virtual representation in order to predict the future state of any pharmaceutical process.
3. Autonomous Robots: robotization of any process leads to many benefits, such as: decreasing the defect rate, providing a higher level of quality and better work space utilization. Usually, people can work with robots on carrying out different tasks using a smart sensor, equipped with a human-machine interface. Robotization has many applications in the pharmaceutical industry; it can be applied in the warehouse management as robots will help to do an automated pick-place step in a vertical manner and a material transfer step in a fast way. Also, a robotic cloud lab can be established to help the researchers carry out the repetitive tasks, while they carry more essential ones. Additionally, automating the filling and packaging operations helps to carry out an automated quality inspection (e.g. to automatically detect visually defected vials or those with a low fill volume quickly). Finally, by Robotic Process Automation RPA, a digital Pharmacovigilance PV Lab can be designed to eliminate the manual tasks related to data collection, tracking and making decisions concerning the safety of the marketed drugs.
4. Internet of Things IoT: this trend includes the use of a system of interconnected devices, equipped with sensors and software, to facilitate the exchange and the gathering of data, which in-turn allows for an effective decision making. For example, IoT monitoring sensors can alert the maintenance supervisor in case of an urgent maintenance problem who will respond to the received alarm promptly and fix it remotely as well. Another beneficial application is the use of IoT temperature and humidity sensors to provide real-time data during the batch transportation process in order to achieve a better supply chain control. Most importantly, IoT will help to switch from Single Batch to Continuous Process Manufacturing CPM as it will create IoT-driven manufacturing operations, such as: measuring on-line analytics (e.g. specifications, metrics) to improve the quality of the product, tracking the assets status (e.g. location, usage) quickly to maximize their utilization in the process, and building a predictive maintenance model to act proactively to the anticipated machinery failures (i.e. to reduce the unexpected downtime), all of these will ultimately lead to an uninterrupted flow of materials.
5. Big Data Analytics BDA: this trend involves the gathering of a massive amount of data (i.e. Big Data) that cannot be analyzed through the traditional means. There are four types of analytics that can be performed using BDA: descriptive to visualize the current process, diagnostic to identify the causes of problems, predictive to anticipate the future business needs, and prescriptive to recommend actions and strategies. There are many examples of the use of Big Data in the biological pharma industry, such as: to analyze a large volume of data that is concerning customers` behaviors toward a marketed product and set an effective marketing strategy accordingly. Also, machine learning algorithms, using techniques including: decision tree analysis, help to recruit patients in large clinical trials in a timesaving manner.
Finally, researches using this trend can utilize the historical data found in clinical trials to build a predictive model in order to analyze the safety and efficacy profiles of a given drug, thus accelerating the new drug discovery and approval processes.
6. Cloud Computing CC: since the use of IoT and Big Data Analytics require sophisticated software and hardware capabilities for data processing, cloud computing (i.e. on-demand availability of data storage and computing centres over the internet) can help to manage this need by performing the data analysis through cloud servers. Eventually, by using a public cloud (i.e. provided by third parties), a private cloud (i.e. resources are provided by a single organization), a hybrid cloud (i.e. a combination of public and private types), and a community cloud (i.e. shared by several organizations), Cloud Computing helps to establish a flexible infrastructure that connects distant manufacturing sites distributed in different geographical areas.
Name of the Author: Noor A. Saleh.
An industrial pharmacist with more than 5 years of experience in the field of pharmaceutical manufacturing, who has a deep GMP knowledge in the following areas: handling of all types of document management activities in alliance with ICH, FDA, Eudralex & PIC/S guidelines, handling of deviations, out-of-specification (OOS) reports, and all types of suppliers’ nonconformities, issuing of Annual Product Quality Reviews (APQRs) and Quality Metrics report, and following up the qualification activities of all new suppliers and reviewing their technical agreements.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


