Neeta RatanghayraMay 31, 2021
Despite advances in anticancer chemotherapy, small-molecule anticancer drugs are associated with a narrow therapeutic window and limited efficacy. A robust strategy for targeted therapy, known as antibody-drug conjugates (ADCs), has been developed to counterfeit this challenge.
ADCs consist of a tumor-specific monoclonal antibody covalently conjugated to a cytotoxic drug using a chemical linker. The cytotoxic drugs (chemotherapeutic agents) linked to the monoclonal antibody are known as cytotoxic payloads or warheads.
ADCs increase the efficacy and reduce the systemic toxicity associated with current chemotherapeutic regimens. ADCs can deliver highly cytotoxic payloads directly to tumor cells, and hence they are highly lethal towards the targeted tumor cells. Due to this selectivity, ADCs are also referred to as the "Trojan Horses."
ADCs use monoclonal antibodies to deliver potent cytotoxic payloads to tumors that overexpress a particular target.
To enable exclusive binding of the monoclonal antibodies (mAb) to its target site, the target antigens should be expressed on the tumor cells but not on normal cells.
Once the ADC recognizes and attaches to its target antigen, the ADC–antigen complex is internalized into the cell through receptor-mediated endocytosis. The ADCs are engulfed into endosomes that eventually mature and fuse with lysosomes.
Due to the acidic environment and the presence of lysosomal proteases such as cathepsin B, the ADC is cleaved and the cytotoxic drug is released into the cytoplasm of tumor cells. Once released, the cytotoxic warheads bind to their target, either the microtubules or DNA, and induce apoptosis, and ultimately cell death.
The success of an ADC is depends significantly on the specific properties of its four components – antibody, antigen, cytotoxic payload and the linker.
Selecting a unique antigenic target for the monoclonal antibody is the most critical step during ADC development. The antigen should be selectively expressed in tumor cells and have a negligible expression in the healthy cell. The unique antigenic target should also have robust internalization properties to promote the transport of ADC into the cell, which, in turn, will increase the efficacy of the cytotoxic agent.
Another vital component is the antibody. Apart from delivering the cytotoxic drug to the tumor cell, the antibody should possess high binding affinity for the tumor cells' antigens. Low immunogenicity, low cross-reactivity, good retention, and adequate linkage-binding capacity are other ideal properties.
The linkers also play a vital role. When the ADC complex is released in the systemic circulation, the linker should remain stable and prevent the release of the cytotoxic payload in the off-target tissue. The linker must keep the ADC in an inactive, nontoxic state; however, upon internalization, the linker should release the cytotoxic drug.
The cytotoxic payloads are activated when they are released inside the tumor cell cytoplasm. The drug should be highly potent and have a small molecular weight. They should possess high stability in the systemic circulation and lysosomes. Low immunogenicity and a long half-life are other essential features of an ideal cytotoxic payload. Moreover, the cytotoxic payload's chemistry should be such that it can be easily conjugated to the linker while retaining the internalization property of the monoclonal antibody.
DNA-damaging agents (Calicheamicin, Doxorubicin) and microtubule-disrupting agents (Auristatin, Maytansinoids) are the two common classes of cytotoxic payload used in ADC development.
The concept of ADCs may seem pretty straightforward; however, developing an optimized and functional ADC is challenging. Optimizing the formula to balance the three components right is the main issue.
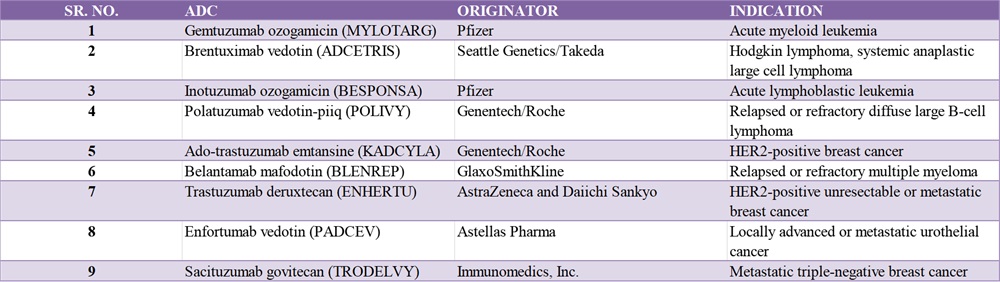
Though many ADC therapies are in the pipeline, only a few have reached the market. Currently, nine Food and Drug Administration (FDA)-approved ADCs are available, and more than 100 others are in clinical studies.
The clinical application of ADCs comes with its own set of challenges, among which the issue of toxicity is the most critical. The toxicity of ADCs is mainly caused by the cytotoxic drugs.
The specificity of antibodies is another issue. Ideally, target antigens need to be tumor-specific and possess high expression in tumor cells and negligible expression in healthy tissues. However, practically, antigens are also expressed in normal tissues.
Current animal models are incapable of predicting the ADCs' activity in humans. Many ADCs demonstrate therapeutic benefits in rodent tumor models; however, these effects are not replicated in the clinic. The difference between rodents and human antigens is said to be the reason for this.
Conducting clinical trials of ADCs is also a challenge, particularly identifying patients who overexpress the targets of interest.
Over the past few years, advances in technologies have generated a range of possibilities to design new ADCs. For example, many novel antigen targets have been identified for both solid and hematologic tumors. Several highly potent cytotoxic drugs have been discovered, such as anthracyclines, microtubule inhibitors, and amatoxins which can be included as suitable complements to currently used cytotoxic drugs. Apart from this, new generation linkers have been characterized to enhance the therapeutic window of ADCs.
Research is ongoing to develop bispecific ADCs. With the use of bispecific ADCs, both potency and selectivity can be improved; multiple classes of payloads can also be delivered. Combination strategies such as combining checkpoint inhibitors and traditional chemotherapies are also being explored.
Although there are many challenges to overcome, the development of new ADCs provides significant opportunities for future cancer therapies.
1.Staudacher AH, Brown MP. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required?. Br J Cancer. 2017;117(12):1736-1742.
2.Barok M, et al. Extracellular vesicles as modifiers of antibody‐drug conjugate efficacy. Journal of Extracellular Vesicles. Available at: https://onlinelibrary.wiley.com/doi/full/10.1002/jev2.12070. Accessed: 30 March 2021.
3.Khongorzul P, Ling CJ, Khan FU, Ihsan AU, Zhang J. Antibody-Drug Conjugates: A Comprehensive Review. Mol Cancer Res. 2020;18(1):3-19.
4.Joubert N, Beck A, Dumontet C, Denevault-Sabourin C. Antibody-Drug Conjugates: The Last Decade. Pharmaceuticals (Basel). 2020;13(9):245.
5.Zhao P, Zhang Y, Li W, Jeanty C, Xiang G, Dong Y. Recent advances of antibody drug conjugates for clinical applications. Acta Pharm Sin B. 2020;10(9):1589-1600.
6.Advances and Challenges in Antibody–Drug Conjugate Development. Available at: https://dailynews.ascopubs.org/do/10.1200/ADN.20.200278/full/. Accessed: 30 March 2021.
7.Hafeez U, Parakh S, Gan HK, Scott AM. Antibody-Drug Conjugates for Cancer Therapy. Molecules. 2020;25(20):4764.
Neeta Ratanghayra is a freelance medical writer, who creates quality medical content for Pharma and healthcare industries. A Master’s degree in Pharmacy and a strong passion for writing made her venture into the world of medical writing. She believes that effective content forms the media through which innovations and developments in pharma/healthcare can be communicated to the world."


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


