Deepak HegdeMay 10, 2021
Tag: Continuous Manufacturing , API
ABSTRACT: This article, which is Part 2 of the series on continuous manufacturing focuses on the uptake of continuous manufacturing by the pharmaceutical industry for both API and drug products, scaleup to pilot scale and successes in commercialization. Adoption of continuous manufacturing by CRO’s/CDMO’s is also reviewed. Challenges associated with continuous manufacturing are outlined.
***Click and get access to the full article***
Continuous manufacturing has been common in certain sectors like the oil and gas for a long time. However pharmaceutical industry has been slow to catch onto this concept and has adapted it only since about 2005 and has produced success, albeit limitedly, at commercial scale in the past decade.
API manufacturing processes typically have more steps and therefore is more complex when compared to solid dosage manufacturing. But with continuous anufacturing’s production efficiencies, interest in and adoption of continuous API manufacturing should increase once approvals comes through.
At a pilot plant level, the first continuous manufacturing plant was demonstrated in 2013 by Mascia et al. when Ali-ski-ren hemi-fuma-rate was synthesized, purified, crystallized, and subsequently formulated into the desired DP while mitigating solids handling issues and removing the need for intermediate solvent swaps between unit operations1. In 2016, Adamo et al. also demonstrated a compact, end-to-end production of multiple DPs with different APIs 2. In 2016, Monbaliu et al. developed an automated system for the end-to-end CPM of lidocaine hydrochloride 3. In 2019, Cole et al. described the end-to-end CPM of merestinib (a new biliary tract cancer drug) from synthesis to crystallization 4, 5 The above-mentioned demonstrations were implemented on pilot or production plant level. In 2017, Eli Lilly’s continuous API GMP facility could produce 3 kilograms a day of prexasertib monolactate monohydrate, a chemotherapy candidate for clinical trials.6 The process links each stage in the process to quality-control systems, combining synthesis with purification and crystallization. It also enables chemistries that would be impossible or too dangerous using traditional methods.
The implementation of continuous manufacturing at production level has begun to appear more prevalently in recent years for plant subsystem and plantwide designs.7-9 Vertex committed to continuous Or-ka-m-bi (containing lumacaftor and iva-caf-tor for cystic fibrosis treatment) tableting.10In 2016, Janssen received FDA approval for the continuous tablet production for Pre-zi-s-ta, whose API, da-ru-na-vir, is used as part of HIV/AIDS combinative treatments.
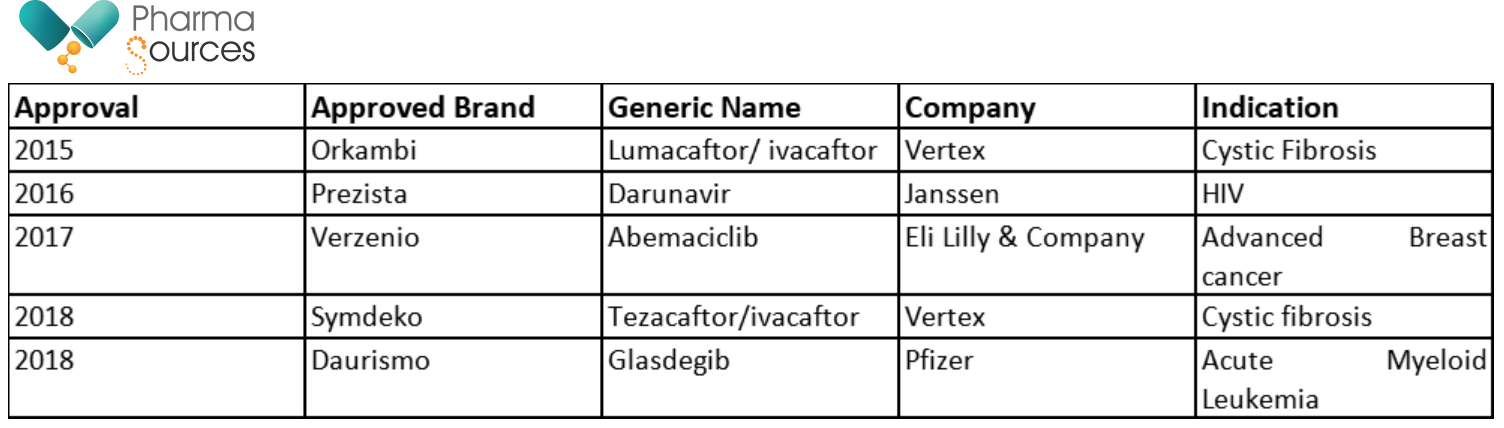
US-FDA has publicly backed continuous manufacturing, and this has also reflected in the product approvals for the US market in Table 1. have been approved by the US-FDA and have been launched in the US market.

GSK began continuous production of amoxicillin at a fully continuous plant in Singapore 12 and also began a continuous line for daprodustat (an anemia medication). 13-14GSK believes that anywhere between one-third and one-half of their drug portfolio could be transitioned to continuous manufacturing.15 In addition to building the Singapore plant for GSK, Zeton, a global equipment and process technology supplier, recently helped design, build, and install a continuous API manufacturing skid for GSK in their Global R&D hub in Upper Providence, PA, USA.16 Eli Lilly recently committed a significant capital investment to a continuous production plant in Ireland.17
Pharmaceutical companies have been outsourcing activities to CRO’s/CDMO’s for capacity or capability reasons. If the pipeline in the pharmaceutical industry starts getting meaningfully skewed towards continuous manufacturing, then CRO’s/CDMO’s will also have to start adapting to this to keep in line with that industrial trend and investing quite significantly in this field.
However, CRO’s/CDMOs face several obstacles18 setting up the building blocks of continuous manufacturing, the most prominent one is the upfront investment for new equipment and the sensors that check for quality as drug product moves along the assembly line as most products require customized processes for every product, even though it is common equipment. Secondly, more than buying the technology, recruitment of automation experts and process scientists, who have experience in handling this technology is key as the pool of such experts is relatively small given the fact the fact that this technology is still relatively new. Most discussions that CDMO’s are having about this technology with clients are more detailed than those for batch production with a majority of them being focused on things like process controls, logistics and skills tests.
Notwithstanding these issues, CDMO’s like Lonza, Catalent, Aesica, Snapdargon Chemistry, and Patheon (now Thermo Fischer) are CMOs and CROs that have invested early in continuous manufacturing technologies, Patheon (now Thermo Fischer) has recently invested in a 50 kg h−1 powder-to-tablet continuous manufacturing system.19,20
Compared to batch manufacturing, there are several challenges associated with continuous manufacturing. Figure 1 outlines some of the challenges associated with continuous manufacturing
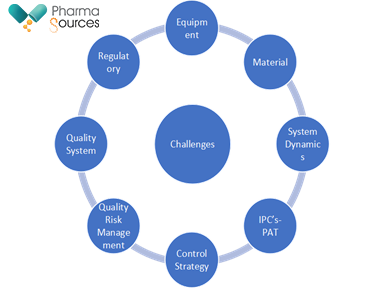
Fig.1: Challenges in continuous manufacturing
One of the key risks in in terms of Quality Risk Management. While the expectation for product quality is the same for a product manufactured by continuous manufacturing when compared with batch manufacturing, some key differences in Quality risk Management are highlighted.
1.Risk assessment: Hazards identified for a continuous manufacturing process are different than for batch manufacturing process. Hence, understanding the process dynamics in relation to process conditions and material properties is the foundation for effective risk management.
2.Risk mitigation: Control strategies may be different for continuous manufacturing process than for batch manufacturing process. Examples include more frequent use of model-based control, multivariate monitoring, analysis of large of data sets, automation and/or Real-Time Release Testing (RTRT)
3.Risk communication: Communicating residual levels of risk is vital. Linking adopted control strategy approaches to risk assessment can be an effective mechanism for communicating product and process development, as well as life cycle management
Table 1: US-FDA Approvals of Drugs Produced by Continuous Processes (At time of writing this article in Mar 21)
Europe has been relatively slower in terms of product approvals using the continuous manufacturing technique as is reflected in the comparatively lower number of approval of products in Table 2.
Table 2: EU Approvals of Drugs Produced by Continuous Processes (At time of writing this article in Mar 21)

Read More: Advantages to Drug Companies to Approach Continuous Manufacturing
1. Mascia, S.; Heider, P.L.; Zhang, H.; Lakerveld, R.; Benyahia, B.; Barton, P.I.; Braatz, R.D.; Cooney, C.L.; Evans, J.M.B.; Jamison, T.F.; et al. End-to-end continuous manufacturing of pharmaceuticals: Integrated synthesis, purification, and final dosage formation. Angew. Chem. Int. Ed. 2013, 52, 12359–12363.
2. Adamo, A.; Beingessner, R.L.; Behnam, M.; Chen, J.; Jamison, T.F.; Jensen, K.F.; Monbaliu, J.-C.M.; Myerson, A.S.; Revalor, E.M.; Snead, D.R.; et al. On-demand continuous-flow production of pharmaceuticals in a compact, reconfigurable system. Science 2016, 352, 61–67.
3. Monbaliu, J.-C.M.; Stelzer, T.; Revalor, E.; Weeranoppanant, N.; Jensen, K.F.; Myerson, A.S. Compact and integrated approach for advanced end-to-end production, purification, and aqueous formulation of lidocaine hydrochloride. Org. Process Res. Dev. 2016, 20, 1347–1353.
4. Cole, K.P.; Reizman, B.J.; Hess, M.; Groh, J.M.; Laurila, M.E.; Cope, R.F.; Campbell, B.M.; Forst, M.B.; Burt, J.L.; Maloney, T.D.; et al. Small-volume continuous manufacturing of merestinib. Part 1. Process development and demonstration. Org. Process Res. Dev. 2019, 23, 858–869.
5. Reizman, B.J.; Cole, K.P.; Hess, M.; Burt, J.L.; Maloney, T.D.; Johnson, M.D.; Laurila, M.E.; Cope, R.F.; Luciani, C.V.; Buser, J.Y.; et al. Small-volume continuous manufacturing of merestinib. Part 2. Technology transfer and cgmp manufacturing. Org. Process Res. Dev. 2019, 23, 870–881.
6. Yirka, Bob. “Eli Lilly Develops Continuous Manufacturing Process for Chemotherapy Drug.” MedicalXpress, 16 June 2017. https://medicalxpress.com/news/2017-06-eli-lilly-chemotherapy-drug.html
7. CPI Works with GSK and AstraZeneca on Pharma Manufacturing. https://www.uk-cpi. com/news/delivering-effective-continuous-wet-granulation-processes.
8. How GSK Launched a Continuous Manufacturing Pilot Plant—And What it Learned. https://www.pharmaceuticalonline.com/doc/how-gsk-launched-a-continuous-manufacturing-pilot plant-and-what-it-learned-0001.
9. Drug Companies Warm Up to Continuous Manufacturing—American Chemical Society. https://www.acs.org/content/acs/en/pressroom/presspacs/2019/acs-presspac-may-1-2019/drug companies-warm-up-to-continuous-manufacturing.html
10. Quality by Design (QbD) for the Continuous Manufacturing of Solid Oral Dosage Forms. http://pqri.org/wp-content/uploads/2015/11/Embiata-Smith.
11. FDA Approves Tablet Production on Janssen Continuous Manufacturing Line. http: //www.pharmtech.com/fda-approves-tablet-production-janssen-continuous-manufacturing -line.
12. GSK Invests a Further $77mil to Enhance Antibiotic Manufacturing Facility in Singapore. http://sg.gsk.com/en-sg/media/press-releases/2015/gsk-invests-a-further-s-77mil-to-enhance-antibiotic manufacturing-facility-in-singapore.
13. Palmer, E. GSK Opens $95M Continuous Production Operation in Singapore. https://www. fiercepharma.com/manufacturing/gsk-opens-130m-continuous-production-facilities-singapore.
14. Bailey, C.K.; Caltabiano, S.; Cobitz, A.R.; Huang, C.; Mahar, K.M.; Patel, V.V. A randomized, 29-day, dose-ranging, efficacy, and safety study of daprodustat, administered three times weekly in patients with anemia on hemodialysis. BMC Nephrol. 2019, 20, 372.
15. A. Witty, Glaxo Use New Technology at India Plant, 2013, http://www.fiercephar manufacturing. com/story/glaxo-use-new-technology-india-plant/2013-11- 18.
16. Pharmaceutical Online, https://www.pharmaceuticalonline.com/doc/how-gsk-launched-a- continuous-manufacturingpilot-plant-and-what-it-learned-0001?vm_tId=2013612&user=7af6e7b1-db3c-46ed-8fac-e02218d89cba&sthash.4tAReGcq. mjjoJ.
17. Meet Eli Lilly and Company—2019 Facility of the Year Process Innovation Category Winner | Pharmaceutical Engineering. https://ispe.org/pharmaceutical-engineering/ ispeak/ meet Eli-lilly and company-2019-facility-year-process-innovation
18. Biopharmadive, https://www.biopharmadive.com/news/cdmo-continuous-manufacturing- technology-pharma-drug-industry/532790/.
19. S. Gloss and S. Heidel, Small-scale Process Design to Facilitate Commercialization of a Wet Granulation Process using Continuous Manufacturing and Bringing Continuous Manufacturing to OSD, Poster presentation at AAPS, San Diego, USA, 2014.
20. K. Congdon, Inside Pfizer’s modular manufacturing pods, http://www.pharmaceuticalonline.com/doc/inside-pfizer’s-modular-manufacturing-pods0001.

Deepak Hegde, Ph.D., M.F.M, is an industrial pharmacist by training. He has a been involved in development and commercialization of both innovative and generic drugs from a very early phase of development to technical transfers for commercial manufacturing sites, for the past 25 years. During his career, he has worked Rhone Poulenc, Novartis (Sandoz), USV Ltd., WuXi AppTec and GSK. He is currently working with EOC Pharma. as Chief Technology officer.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


