PharmaSources/CaicaiMarch 26, 2021
Tag: Lenvatinib , Chia Tai Tianqing , First generic
The marketing application (acceptance No.: CYHS1900381) of Chia Tai Tianqing’s Class 4 generic drug “Lenvatinib Mesylate Capsules” has recently changed to the “Under approval” status, and the drug is expected to be approved for marketing this month, with an indication of thyroid cancer, making Chia Tai Tianqing the pharmaceutical enterprise approved for the first lenvatinib generic in China.
A liver cancer-targeted drug more suitable for Chinese patients
Lenvatinib Mesylate Capsules (lenvatinib, code: E7080, trade name: Lenvima) is a multi-targeted, oral receptor tyrosine kinase (RTK) inhibitor targeting vascular endothelial growth factor receptor (VEGFR) 1-3, fibroblast growth factor receptor (FGFR) 1-4, RET, KIT, and platelet-derived growth factor receptor β (PDGFRβ).
In Sep. 2018, the NMPA approved lenvatinib for the treatment of patients with unresectable HCC who have not previously received systemic therapy, making lenvatinib the second first-line treatment for liver cancer following sorafenib in China; in Nov. 2020, lenvatinib’s second indication was approved for the treatment of radioiodine-131-refractory differentiated thyroid cancer (DTC).
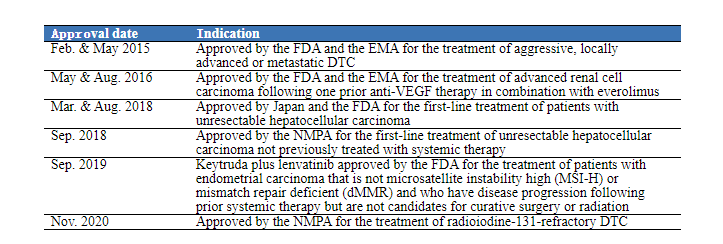
(Organized according to public data)
Lenvatinib can significantly improve overall survival (OS) in Chinese liver cancer patients.
Clinical data on lenvatinib for Chinese liver cancer patients were presented at CSCO in Sep. 2017, according to which, the lenvatinib group had a median OS of 15.0 months, 4.8 months more than the sorafenib group’s 10.2 months.
The main reason for the above difference in efficacy is that lenvatinib is particularly effective in hepatitis B virus-related liver cancer, and hepatitis B virus infection is responsible for over 90% of liver cancers in China. Lenvatinib can be called a targeted drug tailored for Chinese liver cancer patients.
In less than two years following marketing, the sales of lenvatinib in China grew from RMB199 million in 2018 to RMB853 million in 2019, with an annual growth rate of 328.64%, and its sales in China are expected to exceed RMB1 billion in 2020.
80.7% price reduction for entering the NRDL
Lenvatinib was successfully included in the 2020 NRDL (National Reimbursement Drug List of China) in Dec. 2020. The 2020 NRDL has been officially used in China since Mar. 1, 2021, and the prices of the 17 new anticancer drugs in the 2020 NRDL negotiations will all be reduced, to significantly reduce the treatment costs. Among them, the price reduction of lenvatinib is as high as 80.7%, with the price of a single box being only RMB3,240, and reimbursed according to about 70%, the cost of a single box is only RMB972 for individuals (subject to the actual implementation in each region). PharmaSources, one of the healthcare supply companies, could provide you lenvatinib generic of good quality and price. Please contact with us if you have any need of it.
Chia Tai Tianqing to produce the first generic
Eisai’s related patents for lenvatinib include those related to the compound general formula, preparation method, intermediate, crystal form, indication, formulation, and drug combination, with the compound patent to expire on Oct. 19, 2021, the preparation method patent to expire on Nov. 8, 2024 and the crystal form patent to expire on Dec. 22, 2024 in China.
Nine pharmaceutical enterprises have so far applied for the marketing of Class 4 generic drug of lenvatinib in China. Among them, Chia Tai Tianqing applied for the marketing of its lenvatinib generic drug according to the new Class 4 in June 2019 to become the one applying for producing the first generic, and now it will be the first one to produce the first generic.
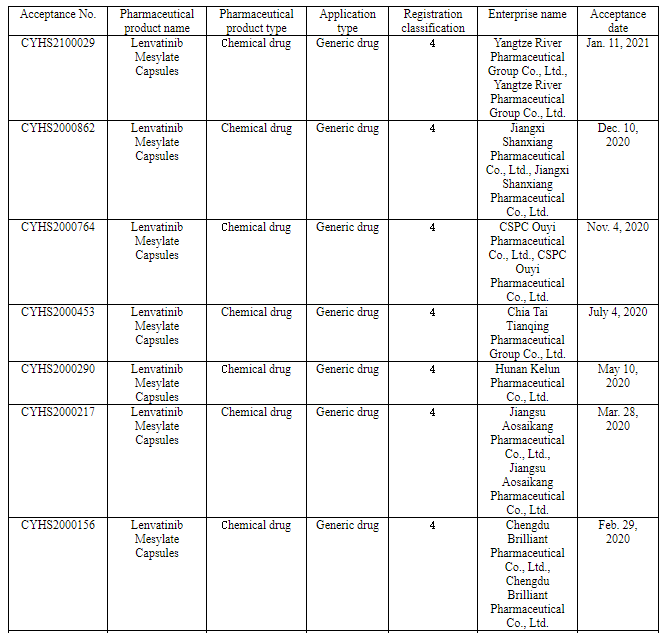
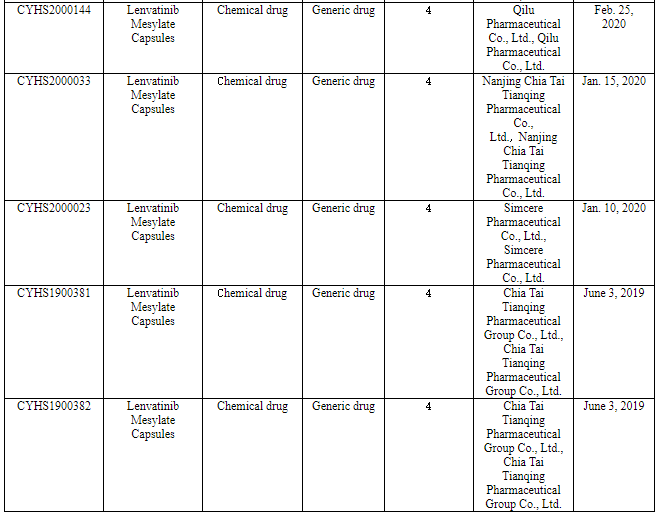
(Source: CDE)
China is a big country in terms of liver cancer, with 466,000 new liver cancer patients every year, 80% of whom are in the middle and advanced stage, and with 422,000 deaths every year, therefore, lenvatinib has a huge market prospect, and its sales will rise in the future. Furthermore, the effects of lenvatinib in combination with anti-PD-1 drugs are amazing, therefore, according to the prediction of the author, Chia Tia Tianqing’s first lenvatinib generic will achieve good sales.
Caicai, a Master of Pharmacy from Shanghai Jiaotong University, used to work in the Institute of Science and Technical Information. Currently as a practitioner in the drug surveillance system, she is good at interpreting industry regulations, pharmaceutical research developments, etc.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


