PharmaSources/XiaoyaowanFebruary 08, 2021
Tag: Vaccine , COVID-19 , china
The COVID-19 pandemic has circulated again recently in Europe and America, with the number of new confirmed cases hitting new highs in countries represented by the UK and the U.S., which has objectively put forward higher requirements for pandemic prevention and control. Urgently in demand, the development of COVID-19 vaccines has been accelerated, and many vaccines have been approved to be vaccinated worldwide. This is expected to relieve the current anti-pandemic pressure and reverse the pandemic to some extent.
R&D progress of COVID-19 vaccines in the world
According to the public information at present, more than 40 COVID-19 vaccines have entered the clinical stage worldwide, of which 17 have entered/completed phase III clinical trials, 8 are in phase II clinical trials, and 18 are in phase I/II clinical trials.
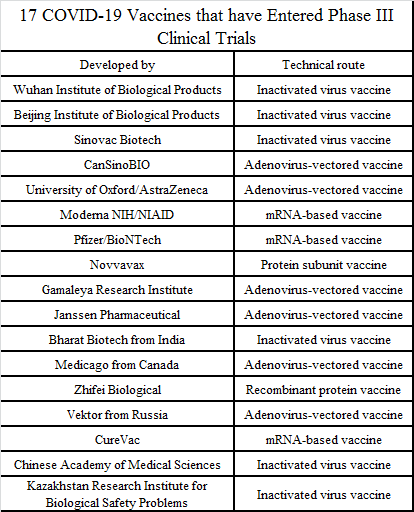
Source: Organized according to public data
Depending on the technical route, the current COVID-19 vaccine varieties in the world include inactivated virus vaccines, adenovirus-vectored vaccines, mRNA-based vaccines, and protein subunit vaccines. Each technical route has advantages and disadvantages: inactivated virus vaccines have the simplest process, however, the immunizing potency is relatively low; adenovirus-vectored vaccines are well tolerated while the anti-adenovirus antibodies in some people can cause the vaccines to fail; mRNA vaccines can be produced fastest, although they are easy to be degraded.
An overview of vaccines approved for marketing worldwide
A total of six COVID-19 vaccines have been approved for marketing worldwide by Jan. 12, 2021, separately, BNT162b2 and mRNA-1273 under the Emergency Use Authorization (EUA) in the U.S., AZD1222 under the EUA in the UK, an inactivated COVID-19 vaccine conditionally marketed by Sinopharm CNBG in China, an inactivated vaccine from India under the EUA in India, and “Sputnik V” approved for marketing in Russia.
The entire development process of a vaccine normally takes 8 to 10 years, with a statistical success rate of only about 20%. According to public data, only six vaccines have been successfully developed and marketed in contrast to 44 newly discovered infectious disease pathogens in the past 50 years, with a success rate of less than 14%. However, the severe global situation of the COVID-19 pandemic since 2020 has prompted the development and approval of COVID-19 vaccines to be greatly accelerated. The COVID-19 vaccines approved for use in China, the U.S., and the UK are all marketed conditionally or under the EUA, and the subsequent monitoring data still need to be taken into consideration for approval.
It should be noted that no valid clinical trial data have been made available for the inactivated vaccine approved under the EUA in India and the “Sputnik V” approved for marketing in Russia, thus their efficacy is doubtful.
Inactivated COVID-19 Vaccine of Sinopharm CNBG
An inactivated virus vaccine is a vaccine that can cause the virus to lose its infectivity and replication ability through physical or chemical treatment while retain the virus’s activity to trigger an immune response in humans. Inactivated vaccines are vaccine products with prototype viruses entirely inactivated, retain almost all the antigens and epitopes of the viruses, and can target the conserved epitopes of viruses to reduce the possibility of virus escape.
The inactivated COVID-19 vaccine of Sinopharm CNBG received approval from the National Medical Products Administration of China (NMPA) at the end of Dec. 2020 for conditional marketing. According to the interim analysis data results of phase III clinical trial, the vaccine had good safety after vaccination, and after two injections of the immunization procedure, all vaccine recipients had high-titer antibodies, with a neutralizing antibody-positive conversion rate of 99.52%. The protective efficacy of the vaccine against the disease caused by the novel coronavirus infection (COVID-19) reached 79.34%, and the data results met the relevant WHO technical standards and the relevant standard requirements in the Guidelines for the Clinical Evaluation of Prophylactic Vaccines against COVID-19 (Trial) issued by the NMPA.
Chinese Vaccines Going Global amid the Receipt of COVID-19 Vaccines Worldwide
The Joint Prevention and Control Mechanism of the State Council of China held a press conference on the recent pandemic prevention and control and vaccination on Jan. 9, according to which, the cumulative number of people reported to have been vaccinated in China reached 7.383 million, plus the key populations vaccinated earlier in different provinces, leading to a total of more than 9 million vaccinated.
Besides China, some countries have also started to conduct COVID-19 vaccination in succession. According to statistics by Jan. 8, 2021, COVID-19 vaccines received worldwide reached 17.3 million doses, involving 18 countries, with the main brands of COVID-19 vaccines involved being Pfizer BioNTech, Sinopharm CNBG, Sinovac Biotech, and Sputnik V; some rich countries with small populations already had vaccination rates of more than 10%, such as the United Arab Emirates (UAE).
It is noteworthy that the COVID-19 vaccine administered in the UAE is the inactivated virus vaccine from Sinopharm CNBG. And besides the UAE, many countries including Bahrain, Egypt, Morocco, and Brazil have received/are planning to receive COVID-19 vaccines from China. According to the Phase III clinical trial data of Sinovac’s COVID-19 vaccine announced in Brazil on Jan. 8, it achieved a 78% effective rate and 100% effectiveness in severe cases.
Compared with mRNA vaccines that require long-term storage at -70°C, Chinese vaccines require milder storage conditions and do not have harsh requirements for cold chain pharmaceutical logistics and other facilities, leading to a lower overall cost and easy acceptance by most countries. And there is another way for other countries to obtain Chinese vaccines: direct production locally by obtaining licenses and conducting cooperation. This “cooperative production” method with Chinese characteristics can shorten the transportation distance of vaccines, further reduce vaccine transportation and storage costs, and facilitate local production and vaccination in other countries.
From the perspective of global population distribution, developing countries, which account for 80% of the population, have the most urgent demand for vaccines under the impact of this pandemic, and most of these countries do not have the overall strength of COVID-19 vaccine development and industrialization. The Chinese-produced COVID-19 vaccines take on the role of global public goods and will soon provide strong support for the safety protection of these countries.
References:
1. CCTV News
2. National Health Commission of China
3. Guosheng Securities Research
Xiaoyaowan, a pharmaceutical industry practitioner, a word carrier in the We-media era focusing on changes of the pharma industry.、


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


