The world is full of wonders. According to an article published by a British doctor in the British Journal of Haematology two days ago, a malignant lymphoma patient’s tumor almost disappeared after infection with coronavirus! It was a 61-year-old man with severe kidney disease and requiring long-term dialysis treatment to sustain life; not long ago, he was hospitalized for treatment of lymphadenopathy and weight loss and was found to have advanced Hodgkin lymphoma, with PET/CT examination showing active cancer cells everywhere in his body (the black areas in the figure on the left below represent the ones taken over by tumor cells).
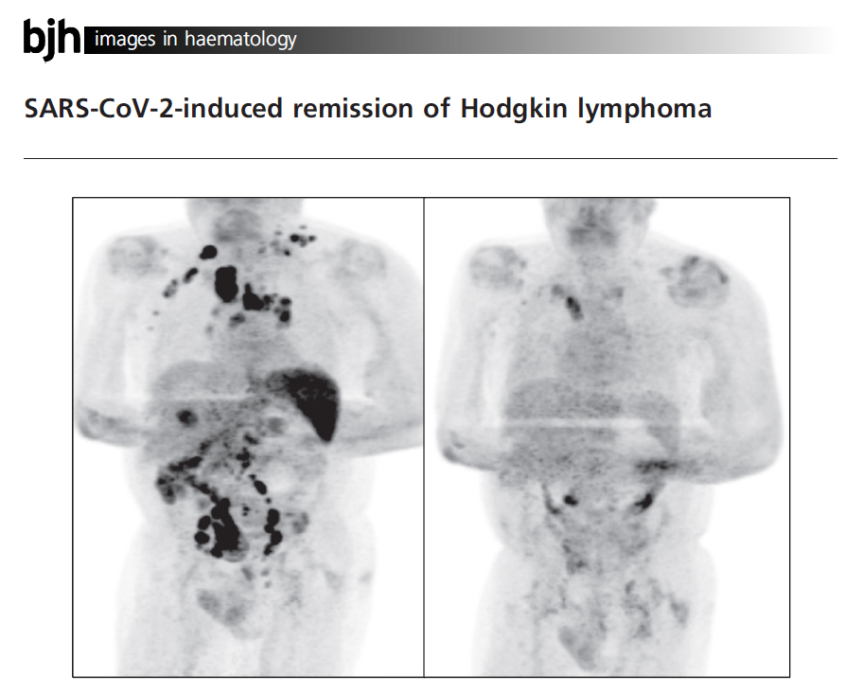
Changes in Tumor Foci Before and After Infection with Coronavirus (Source: Reference 1)
To add insult to injury, he was hospitalized again for breathlessness shortly after diagnosis, and this time he was diagnosed with COVID-19. His symptoms remitted 11 days after hospitalization, and he was discharged to convalesce at home. No corticosteroid or immunochemotherapy was administered during the period.
Miraculously, four months later, the PET/CT reexamination revealed that most of the tumor in his body disappeared (see the figure on the right above, where the black areas disappear) and the levels of tumor-related biomarkers dropped by more than 90%. What happened? Researchers currently have no clear conclusions as to this phenomenon, and the mainstream hypothesis is that the coronavirus infection accidentally triggered an anti-tumor immune response in the patient’s body, which not only killed the virus but also destroyed the cancer cells in the process.
This phenomenon is not an isolated case, and there were cases of patients with other cancers whose tumors disappeared after they were infected with viruses in history. As a result, researchers have developed a new therapy for tumors based on the phenomenon – oncolytic viruses. Let’s look at it today.
Immunotherapy that “kills cancer with the virus”
Oncolytic viruses (OVs) are a type of viruses selectively infecting and killing tumor cells, with a specific replication ability and the ability to stimulate the body to produce an anti-tumor immune response. OV therapy refers to the use of viral self-replication in infected cancer cells to destroy the host cells, i.e., the use of the original direct cell-killing efficacy of viruses to achieve treatment goals.
By hijacking the protein synthesis “factories” of tumor cells, OVs prevent cells from producing host products and promote cells to produce viral products, which may also be due to the essence of viral virulence; infected host cells undergo lysis to release many viruses with the ability to infect other cells, thus amplifying and spreading the initial infection. However, OVs do not replicate themselves within normal cells, therefore, normal cells are not harmed thereby.
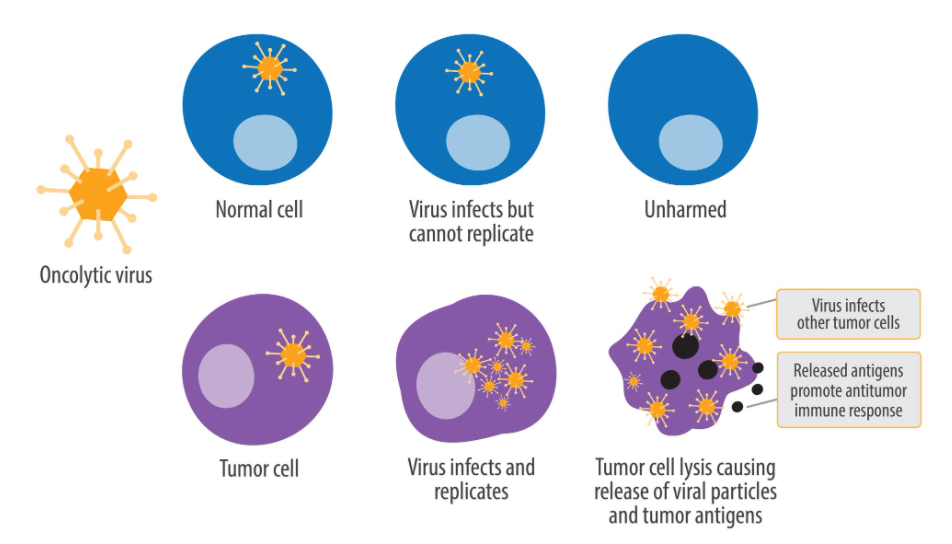
OV Specifically Killing Tumor Cells (Source: Wikipedia)
The world’s first OV was reported because a cervical cancer patient’s tumor regressed after she was infected with the rabies virus. Scientists have made significant progress in OV research in recent years. OVs, as a kind of emerging anti-tumor therapeutic agents, have the advantages of high efficiency in killing tumor cells, good targeting, high safety, small side effects and low cost. OV therapy has been classified as an important branch of tumor immunotherapy as OVs can promote the triggering of the anti-tumor specific immune response in patients while infecting and killing cancer cells.
OVs play a central role in OV therapy. Dozens of viruses have been used for OV therapy to date, including herpes simplex virus type 1 (HSV-1), adenovirus, reovirus, Newcastle disease virus, poliovirus, Coxsackievirus, measles virus, human immunodeficiency virus, mumps virus, vaccinia virus, vesicular stomatitis virus (VSV), and influenza virus.
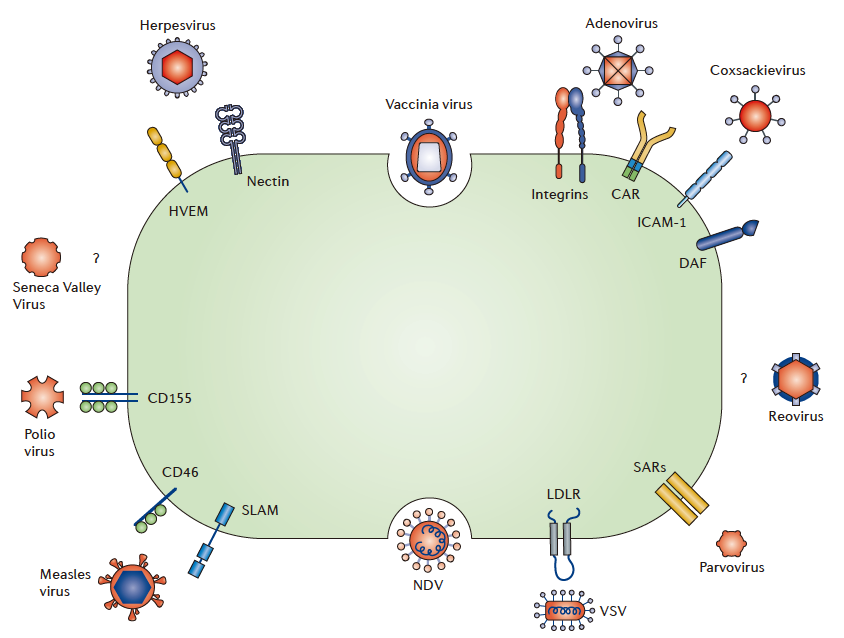
Methods of Different Viruses to Enter Cancer Cells (Source: Reference 2)
Current situation of OV therapy
According to public data, at least three OV therapies have been approved for marketing worldwide, separately, Rigvir by Latima from Latvia, Oncorine by Shanghai Sunway Biotech, and Imlygic by Amgen. Among them, Imlygic is a genetically modified HSV-1 that can replicate and express the granulocyte-macrophage colony-stimulating factor (GM-CSF) in tumor cells. Imlygic was approved in Oct. 2015 in the U.S. for the local treatment of advanced melanoma that has recurred after surgery, to become the first OV therapy approved by the FDA for marketing.
As the only OV drug marketed in China at present, Oncorine is mainly indicated for inoperable liver cancer and liver cancer end-stage ascites and pleural effusion, however, its market size is small as limited by the intratumor injection delivery method.
Rigvir, a genetically modified ECHO-7 enterovirus developed by Latima from Latvia, was approved for the treatment of melanoma in Latvia in 2004. The drug has been approved in Poland, Armenia, etc. Clinical cases over the past decade have demonstrated that Rigvir OV is safe and effective, can increase the survival rate of melanoma patients by four to six times, and has obvious efficacy in solid tumors, including gastrointestinal tumor, pancreatic cancer, bile duct cancer, and sarcoma.
Guizhou Sinorda Biomedicine signed a cooperative development agreement with Latima in Nov. 2019 during the Second China International Import Expo to obtain the exclusive rights of Rigvir in Greater China.
Internationally, OV therapy is sought after by global pharmaceutical enterprises. According to public data, Bristol-Myers Squibb (BMS), Johnson & Johnson, Merck Sharp & Dohme (MSD), AbbVie, Takeda, etc. have been involved in OV therapy development by acquisition or cooperation.
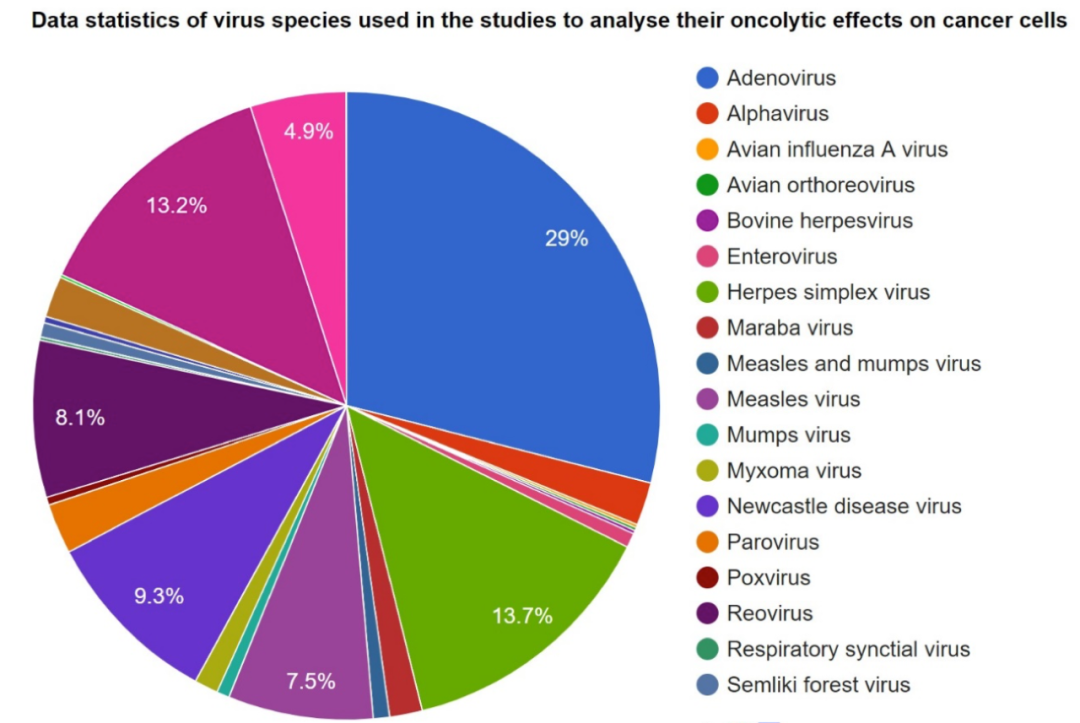
OV Drugs in Development at Present (Source: OvirusTdb)
According to the above figure, in terms of global layout, the R&D of adenovirus drugs still dominates, the R&D of herpes simplex virus drugs and bovine herpesvirus drugs are gaining momentum, and the R&D of other virus drugs is also being conducted.
Chinese enterprises joining the R&D
Many Chinese enterprises and health equipment companies have also joined the R&D of OV therapies, including Sunway Biotech, Tiandakang Gene, Doublle Bioproduct, Kanghong Pharmaceutical, Immvira, Binhui Biotech, CNBG-Virogin, Lepu Biotech, etc. Besides the above approved OV therapy: Oncorine, Sunway Biotech has also developed H102 (tumor-targeted recombinant adenovirus injection) and H103 (oncolytic recombinant adenovirus injection) based on it.
Clinical applications of OVs
The efficacy of OV treatment alone is limited, thus it is necessary to combine OVs with other treatment means for oncology treatment.
OVs in combination with targeted drugs
Three main varieties of targeted drugs have been reported to synergistically enhance OV therapies: epigenetic drugs, PI3K/Akt/mTOR inhibitors, and receptor tyrosine kinase inhibitors.
Among them, the Akt inhibitor Tricibine can work together with the OV MG18L to induce glioma cell apoptosis, and the combination of the two is significantly more effective than monotherapy in treating glioma in mice. Rapamycin, an mTOR inhibitor, can work together with adenovirus and HSV-1 to kill insusceptible tumor cells.
OVs in combination with chemotherapeutics
The combination of OV therapies and chemotherapies can enhance tumor cell antigenicity or susceptibility to immune cells and suppress negative regulatory Treg cells and myeloid-derived suppressor cells (MDSC).
Cyclophosphamide has been reported to be able to enhance the replication and spread of the HSV-1 OV in tumors by significantly inhibiting the body’s innate immune response and neutralizing antibody production and thus expand the replication and killing effects of the OV. Gemcitabine can synergistically enhance the anti-tumor activity of OVs, including adenovirus, reovirus, and vaccinia virus.
OVs in combination with immune checkpoint inhibitors
Immune checkpoint inhibitors are completely dependent on the number of tumor-infiltrating T lymphocytes in tumor patients, while OVs’ infection of tumor cells can induce a large number of tumor-infiltrating immune cells, therefore, the combination of the two has good complementarity.
According to a phase I clinical trial of Amgen’s T-VEC OV and BMS’ anti-CTLA-4 antibody in patients with advanced malignant melanoma, the combination could greatly improve the objective response rate to tumor therapy (combination therapy: 58% vs. monotherapy: 26.4%).
Furthermore, OVs can be combined with other immune checkpoint inhibitors such as PD-1 and TIM-3 (T -cell immunoglobulin and mucin-domain containing-3) inhibitors to further magnify the effect of monotherapy.
OVs have various species and varied regulatory means and can serve as vectors to express exogenous genes with different functions. And OV therapies have better therapeutic effects in tumor treatment. OVs, just like boosters, have a promising future for clinical application in the oncotherapy area.
References:
1.Challenor and Tucker (2021). SARS‐CoV‐2‐induced remission of Hodgkin lymphoma. BJ Haem, https://doi.org/10.1111/bjh.17116.;
2.https://www.nature.com/articles/nrd4663;
3.Another layer of protection. Retrieved January 7, 2021, from https://www.nature.com/articles/d42859-020-00018-3;
4.Pan Haijiao, et al. Oncolytic Virus and Cancer Therapy, Chinese Bulletin of Life Sciences, 2016.





 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story

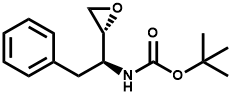
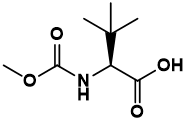

























 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








