Tim FreemanJanuary 18, 2021
Tag: Powder Testing , Amorphous Solid Dispersions , Processing Characteristics
The use of amorphous solid dispersions (ASDs), in which the active(s) is dispersed in a hydrophilic, water soluble excipient or matrix, is a widely recognised strategy for enhancing the bioavailability of poorly soluble drugs. However, the resulting materials are often associated with poor manufacturing efficiency. More specifically, flow properties and compressibility can be less than optimal for high throughput tableting. ASDs can be made by different processing routes including spray drying (SD) and hot melt extrusion (HME) to produce compositionally identical dispersions with substantially different physical characteristics. There is therefore scope to control processability, within the constraints of increasing bioavailability and ensuring adequate stability.
This note summarises work by researchers at the Bernal Institute, University of Limerick, Ireland to address the issue of characterising ASDs to rationalise and predict differences in downstream processing performance1. In a detailed study the morphology and flow properties of ASDs produced by SD and HME were measured and compared to identify differences with the potential to impact processing behaviour. The results highlight the value of dynamic, shear and bulk powder testing for comprehensive, process relevant ASD characterisation.
Experimental Method: Preparing ASD’s
Ternary ASDs of itraconazole (ITZ - Xi’an Liphar Biotech Ltd, Xi’an City, China), Soluplus® (BASF, Ludwigshafen, Germany) and HPMCP HP-55 (hypromellose phthalate - Shin Etsu, Chiyoda, Japan) were produced by SD and HME.
SD was carried out using a 30-40-30 w/w ITZ-Soluplus-HPMCP in dichloromethane-methanol solution, with a lab-scale spray dryer (Büchi B290, Essen, Germany). A 10% w/v concentration solution was used with a 0.7mm spray nozzle. All processing conditions were derived with reference to previous trials and the resulting powder was immediately transferred to a stainless-steel pan and then stored overnight in a vacuum oven to ensure the complete removal of residual solvent. Subsequent powder storage was in a sealed glass bottle, in a desiccator, over anhydrous molecular sieves.
To produce the ASD by HME, HPMCP was initially passed through a 435µm sieve to remove larger particles. A manually premixed 30-40-30 w/w ITZ-Soluplus-HPMCP blend was then fed into a twin-screw extruder (Three-Tec GmbH, Seon, Switzerland). The extruder heating zones, from feed to die, were controlled at 80, 110, 120, 140, 150 and 150°C; screw speed was 15 rpm. Again, these and all other processing conditions were set on the basis of previous trials. The resulting extrudate was milled for 1 min (Retsch Mixer Mill MM 400, Haan, Germany) and then sieved to produce a 90 – 435µm and <90µm fraction each of which was stored separately, under conditions identical to those used for the SD samples.
Samples of the as received ITZ, Soluplus, HPMCP, pre-extrusion raw material mix (physical mix), and prepared ASDs were subject to scanning electron microscopy (SEM – Jeol CarryScope JCM 5700, Tokyo, Japan) and powder testing (FT4 Powder Rheometer®, Freeman Technology, Tewksbury, UK) – dynamic, shear and bulk property measurement - using the standard test protocols for the instrument2. Compaction simulation, true density measurements, formulation/tableting and in vitro dissolution testing were also conducted. However, this note is limited to a discussion of the differences in morphology and powder properties between the samples, and their potential impact on in-process behaviour. Please see reference 1 for full details of the complete study.
The Impact of ASD Preparation Method: (1) Particle Morphology
SEM images show the very different particle morphologies of the three starting materials (Figure 1 A – D). The itraconazole has a relatively flat, wedge-like morphology, with crystal dimensions in the region of around 40 µm by 10µm. Soluplus, on the other hand, has more regularly shaped, almost spherical granules with a diameter of around 250µm. The HPMCP particles have an elongated, cocoon or pod-like structure with a smooth but broken surface; particle dimensions are in the region of 350 µm by 70µm. Images of the physical mix highlight the fineness of the itraconazole compared with the coarser excipients.
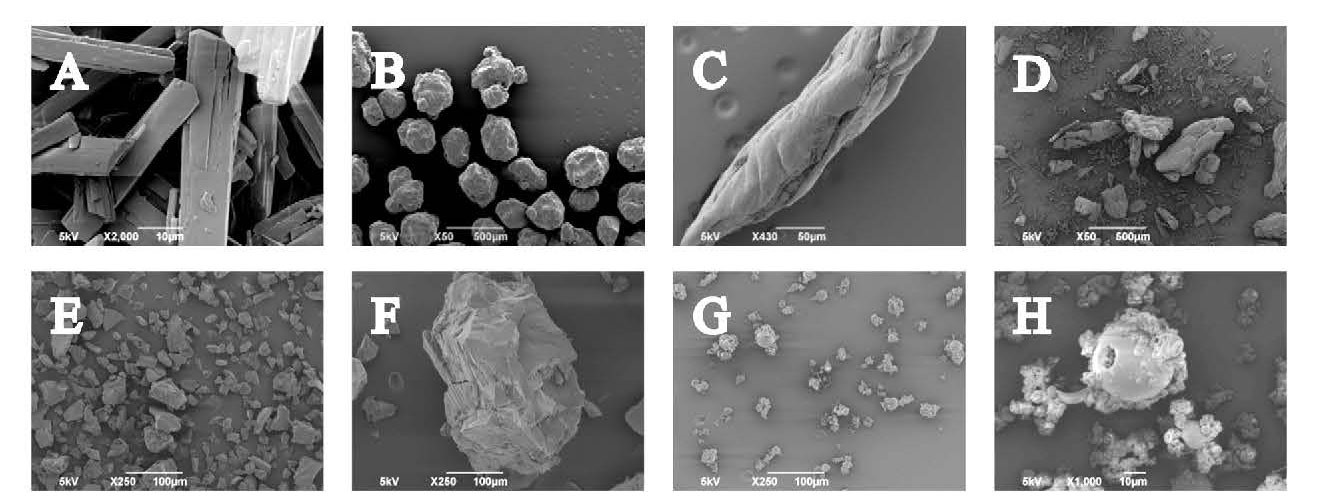
Figure 1 - SEM images of A to D, raw ingredients: itraconazole (x2000),
Soluplus (x50), HPMCP (x430), physical mix (x50) and E to H, ASDs:
HME&BM <90µm fraction (x250), HME&BM 90 – 450 µm fraction (x250), SD (x250) and SD (x1000)
Clear differences in morphology are also evident between the two ASDs. HME, followed by ball milling (HME&BM), produces somewhat irregularly shaped granules with appreciable levels of surface roughness (Figure 1, E and F). Typical dimensions of those in the finer fraction are around 50 by 55µm; for the coarser fraction these increase to around 240 by 350µm. In contrast, SD produces fine hollow spheres. The SD sample consists of spheres up to 30µm in diameter and burst spheres of comparable diameter but mostly contains agglomerates around 40 – 60µm in size made up of crumpled spheres, less than 10µm in size (Figure 1, G and H). Finer particles are clearly advantageous from the perspective of dissolution but are often associated with poor flowability and sub-optimal processing performance.
The Impact of ASD Preparation Method: (2) Dynamic Powder Properties
In dynamic testing values of flow energy are generated by measuring the axial and rotational forces acting on a blade, or impeller, as it is precisely rotated through a powder sample. Stability, flow rate sensitivity and aeration sensitivity were all assessed by measuring dynamic properties for each of the raw materials, the physical mix (no aeration data) and for both ASDs using the 25 ml test vessel2.
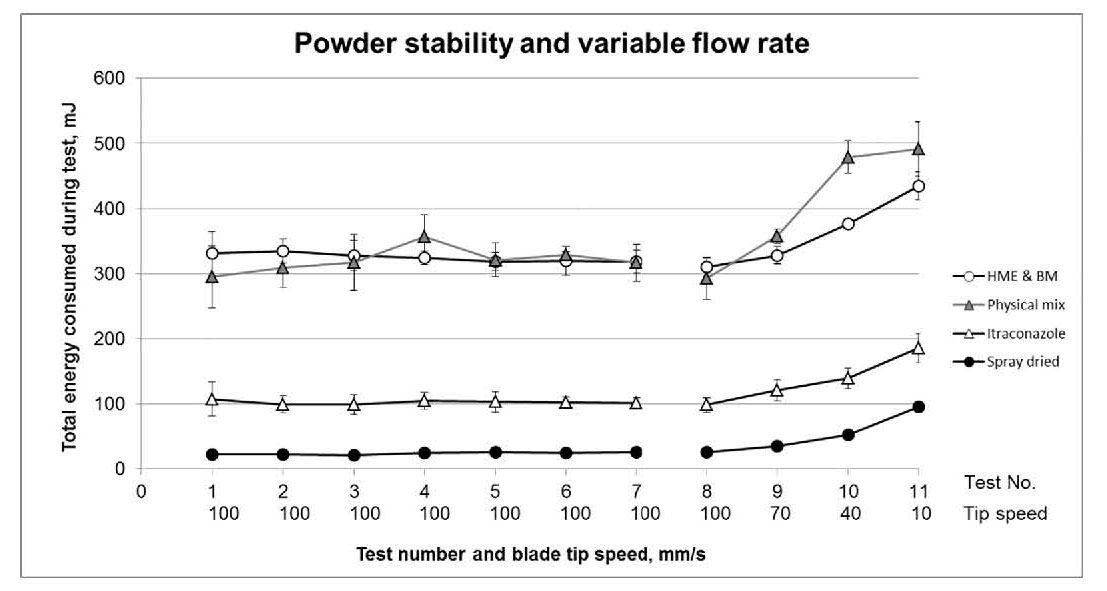
Figure 2 - Stability and Variable Flow Rate testing highlights how the flow properties
of the HPMCP, and especially the Soluplus, change with repeat testing,
and the sensitivity of the SD ASD to flow rate.
Stability Index (SI) values close to 1 indicate that the itraconazole, the physical mix and both ASDs are relatively stable (see figures 2a and b). The Soluplus, and to a lesser extent the HPMCP are less stable with SI values of 1.91 +/-0.35 and 1.35+/0.17 respectively (full data plots not shown). Since both materials are polymeric this instability may be associated with static charge accumulation.
All the materials tested exhibit Flow Rate Index (FRI) values in the 1.5 to 3.0 range except for the SD ASD sample which has an FRI of 3.73 (See Figure 2a and b). FRI values >3.0 are often associated with more cohesive powders so this result is consistent with the fine particle size of the SD sample. It clearly differentiates the SD ASD from the HME&BM ASD which has an FRI of 1.40 and suggests that the two materials would exhibit significantly different responses to changes in flow rate during processing. This observation is particularly relevant for unit operations such as blending and feeding, where performance has been shown to correlate directly with this parameter3.
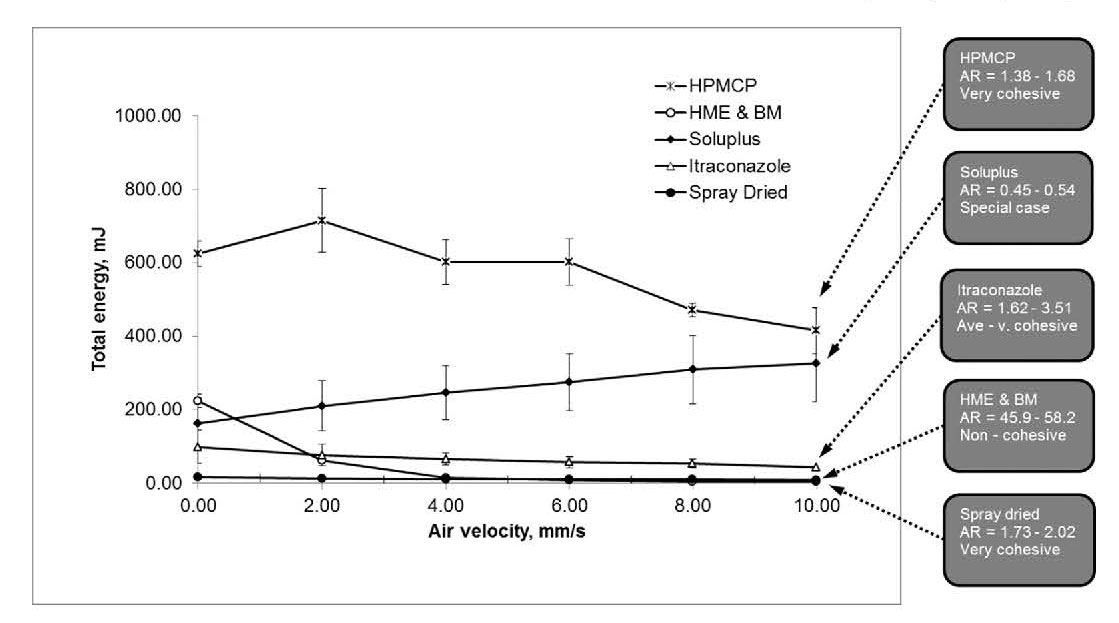
Figure 3: Aeration testing further differentiates the two ASDs
with the flow energy of the relatively non-cohesive
HME&BM sample significantly impacted
by aeration while that of the SD sample is substantially less affected.
Aeration testing at air velocities up to 10 mm/s reveals that the raw materials and ASDs exhibit diverse responses to the introduction of air (see figure 3). Itraconazole has an Aeration Ratio (AR) value in the range 1.62 – 3.51 which is towards the less sensitive end of the typical range (values of between 2 and 20). In more cohesive powders the strength of inter-particulate forces inhibits the passage of air around individual particles, preventing the lubricating and fluidising effects that reduce flow energy. As a result, more cohesive powders tend to be relatively unchanged by aeration and exhibit low AR values. The fine particle size of itraconazole and wedge-like morphology mitigate towards relatively strong inter-particulate interactions and inhibited particle-particle movement and therefore provide a rationale for the observed AR.
HPMCP and Soluplus both exhibit low AR values, of 1.38 – 1.68 and 0.45 – 0.54 respectively, indicating that neither are impacted by aeration to any appreciable extent. With the HPMCP, the size and shape of the particles will result in relatively inefficient packing, indeed, bulk density data confirm this (see below). Any tendency towards horizontal stacking increases the potential for upward thrust on the particles but the open packing arrangement will allow air to transit easily through the powder. In a strictly analogous way, the relatively large, but far more regularly shaped Soluplus granules are also likely to produce a highly permeable powder bed with particle mass further reducing any potential impact of aeration. With this excipient the observed increase in flowability may in fact be associated with the accumulation of electrostatic charge, rather than any influence of aeration.
The SD ASD sample also exhibits a low AR, 1.73 – 2.02. Here, the low sensitivity to aeration can be attributed to high inter-particulate forces of cohesion given the size and surface texture of the particles present (many particles lie in the <10µm range) and their clear tendency towards agglomeration. In contrast, the AR of the HME&BM is very high, 45.9 to 58.2. This test was performed prior to sieving of the sample which consequently has a relatively broad particle size distribution, centred on a much larger particle size than the SD sample. With this material, air flows up and between each individual particle lubricating particle-particle interactions, enhancing flowability, and triggering fluidisation which is accompanied by a dramatic reduction in flow energy. The significant difference in response to air between the ASDs has major implications for pharmaceutical processes such as Würster coating, a fluidised bed microencapsulation process, for other pneumatic operations, and for tableting. The die filling efficiency of a tableting blend correlates directly with its response to air, since air must escape out of the die through the powder for effective filling.
The Impact of ASD Preparation Method: (3) Shear Properties
While dynamic testing has become established as the most informative powder testing technique for process-related studies, shear cell testing remains especially relevant for rationalising powder behaviour under moderate to high stress ranges and for optimising hopper performance. The measurement of shear properties therefore usefully complements dynamic powder testing. Shear cell properties are generated by measuring the forces required to shear one consolidated powder plane relative to another.
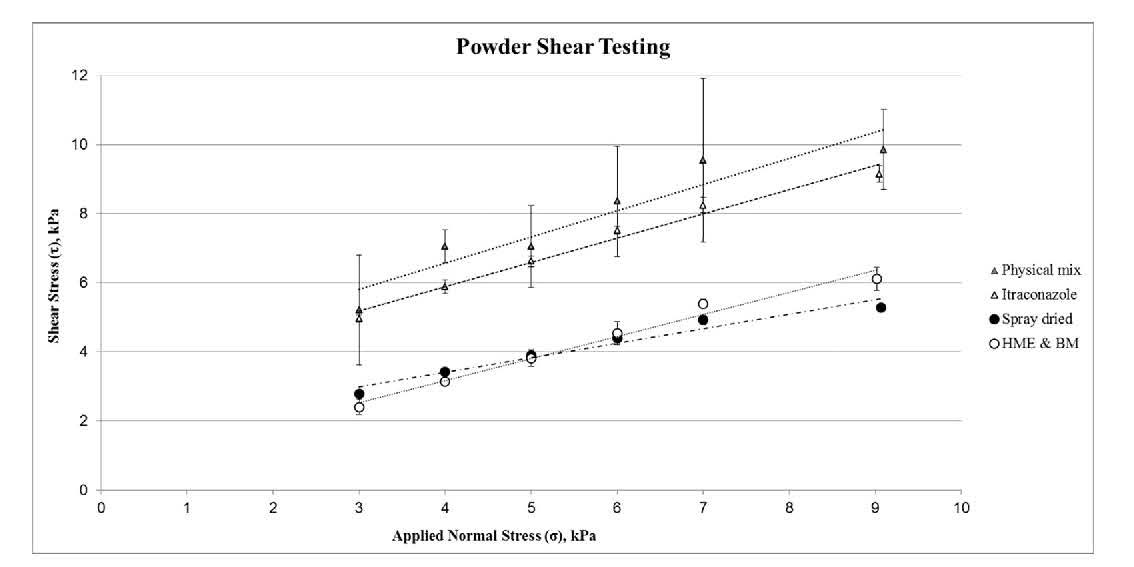
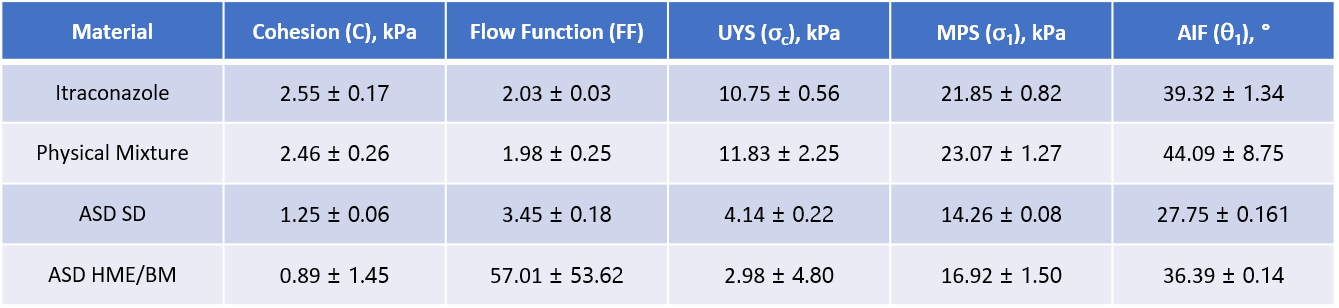
Figure 4/Table 1: Shear testing characterises powder
behaviour under moderate to high stress and is particularly relevant
for the elucidation and control of hopper performance.
Shear cell testing of the itraconazole, the physical mix and each ASD was carried out using the 1 ml and 10 ml test vessels.
Yield locus plots and shear parameters for itraconazole, the raw material blend and both ASDs are reported in Table 1. The itraconazole has a Flow Function Coefficient (ffc or FF) of 2.03 +/- 0.03 while that of the raw material blend is 1.98 +/-0.25. Both materials therefore lie at the cohesive/highly cohesive boundary (an ffc of 2). An ffc of 3.45 +/- 0.18 classifies the SD as cohesive while the HME&BM sample, in contrast has a significantly higher ffc, of 57.01 +/-53.62, indicating it is free-flowing. However, repeatability, while excellent for the cohesive powders is notably lower for the more free-flowing HME&BM ASD sample, a recognised challenge for shear cell testing.
Unconfined Yield Strength (UYS) values rank the materials similarly in terms of flowability, since higher UYS values are generally associated with more cohesive powders. Angle of Internal Friction (AIF), which is the slope of the yield locus, (see figure 4) tends to be indicative of how sensitive the powder is to increasing levels of consolidation, with higher angles associated with greater sensitivity. The two raw materials exhibit the highest AIF values but that of the HME&BM ASD (44.09+/-8.75o) is substantially higher than that of the SD sample (27.75 +/-0.61o). This difference is particularly relevant to pharma primary packaging, shipping and hopper discharge behaviour.
The Impact of ASD Preparation Method: (4) Bulk Density
Alongside dynamic and shear properties, bulk powder properties such as density, permeability and compressibility can be useful in providing a more complete understanding of powder behaviour. Furthermore, changes in bulk density induced by tapping are a simple, traditional way of quantifying flowability. To complete the characterisation of the samples ‘conditioned’ bulk density values were generated for all three raw materials and the two ASDs using the standard protocols for the FT4 Powder Rheometer. These were compared with a single ‘measured’ bulk density value for each powder generated by simply pouring samples from their storage containers into a pre-weighed graduated cylinder. Both values were then used to generate the tapped density parameters Hausner Ratio (HR) and Carr’s Index (CI – also known as Carr’s Compressibility Index)4.
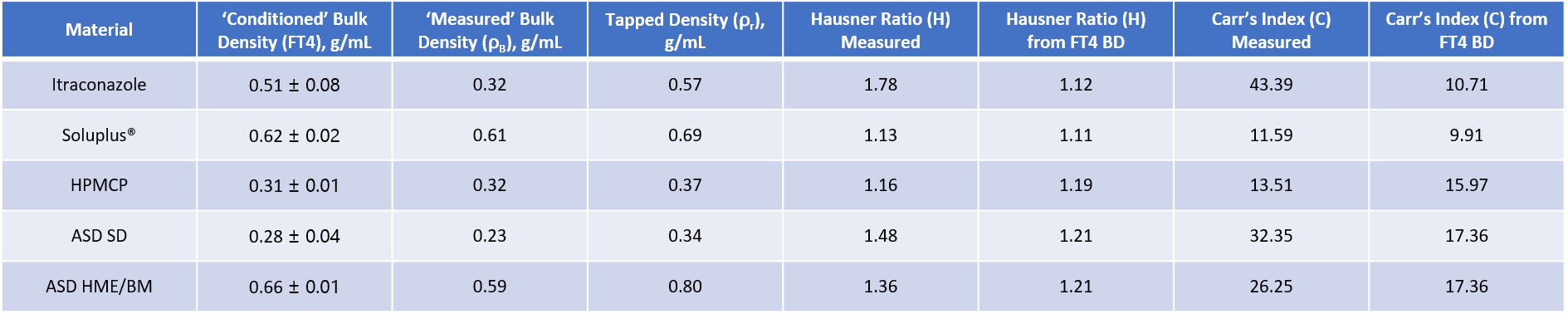
Conditioning involves gentle agitation of the powder bed via a downward traverse of the blade of the instrument, leaving the sample in a homogeneous, loosely packed state. This gives rise to highly repeatable measurements as evidenced from the ‘conditioned’ bulk density data. In terms of the absolute values measured, the HPMCP and SD ASD have the lowest bulk densities. In the case of the HPMCP this can be attributed to the relatively inefficient packing of quite large, irregular particles, as discussed earlier. With the ASD SD, high inter-particulate forces of cohesion between the fine particles give rise to structure in the bed that entrains air thereby resulting in low bulk density.
In general terms the ‘measured’ bulk density values are in reasonable agreement with the conditioned values but there are some important discrepancies, most notably in the values for the itraconazole, which has been identified both as cohesive and sensitive to consolidation. This highlights how easily certain types of powder can entrain and/or lose air, changing bulk density, an effect over which the ‘measured’ bulk density technique offers no control. Both the ASD samples have a significantly lower ‘measured’ bulk density, relative to the conditioned value, suggesting that a degree of deaeration and rearrangement into a consistent, more efficient packing state may occur with these samples too.
An important point to be drawn from these data is that although the bulk density values provide useful insight into the characteristics of the powders, the tapped density ratios do not sensitively differentiate the two ASDs. At best they suggest that the ASD HME&BM has poor flowability (Hausner Ratio 1.35 to 1.45) while that of the ASD SD is very poor (Hausner Ratio 1.46 to 1.59). This is in contrast to both the shear and dynamic data which indicate that these samples are likely to exhibit substantially different performance in certain unit operations. These results demonstrate the potential for variability in unconditioned bulk density measurements, the associated uncertainty of tapped density methods and their inability to reliably differentiate powders.
In Conclusion
The results presented here show how dynamic, shear and bulk properties in combination can be used to make a robust assessment of the processing performance of ASDs. At the same time, they highlight the limitations of using shear data alone or relying on traditional tapped density methods. While shear cell testing is useful for certain applications it is significantly less reliable for less cohesive materials and/or those containing large particles. Tapped density methods can deliver highly variable results, depending on the initial state of the sample and the method applied, and, like shear testing, do not directly quantify the response of a powder to air or changes in applied shear rate for example. Dynamic testing, in contrast, directly quantifies these characteristics providing highly valuable, process-relevant data. Such information directly supports efforts to use ASD technology to achieve high levels of bioavailability without sacrificing process efficiency.
References/Further Reading
1 M. Davis, C. Potter and G. Walker, ‘Downstream processing of a ternary amorphous solid dispersion: The impacts of spraying drying and hot melt extrusion on powder flow, compression and dissolution’ International Journal of Pharmaceutics, 544 (2018) 242–253
2 R. Freeman, ‘Measuring the flow properties of consolidated, conditioned and aerated powders — A comparative study using a powder rheometer and a rotational shear cell,’ Powder Technology, 174 (2007) 25-33.
3 T. Freeman and B. Armstrong ‘Using powder characterisation methods to assess blending behaviour’ Whitepaper available to download at: https://www.freemantech.co.uk/_news_view/using-powder-characterisation-methods
4 <USP 1174> Powder Flow Available to view at: https://www.usp.org/sites/default/files/usp/document/harmonization/gen-chapter/g05_pf_30_6_2004.pdf
Author Biography - Tim Freeman, Managing Director, Freeman Technology

Tim Freeman is Managing Director of powder characterisation company Freeman Technology for whom he has worked since the late 1990s. He was instrumental in the design and continuing development of the FT4 Powder Rheometer® and the Uniaxial Powder Tester. Through his work with various professional bodies, and involvement in industry initiatives, Tim is an established contributor to wider developments in powder processing.
Tim has a degree in Mechatronics from the University of Sussex in the UK. He is a mentor on a number of project groups for the Engineering Research Center for Structured Organic Particulate Systems in the US and a frequent contributor to industry conferences in the area of powder characterisation and processing. A past Chair of the American Association of Pharmaceutical Scientists (AAPS) Process Analytical Technology Focus Group Tim is a member of the Editorial Advisory Board of Pharmaceutical Technology and features on the Industry Expert Panel in European Pharmaceutical Review magazine. Tim is also a committee member of the Particle Technology Special Interest Group at the Institute of Chemical Engineers, Vice-Chair of the D18.24 sub-committee on the Characterisation and Handling of Powders and Bulk Solids at ASTM and a member of the United States Pharmacopeial (USP) General Chapters Physical Analysis Expert Committee (GC-PA EC).


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


