PharmaSources/XiaoyaowanDecember 08, 2020
Tag: Anti-PD-L1 antibody , subcutaneous injection
A Biologics License Application (BLA) for the anti-PD-L1 antibody: envafolimab injection co-developed by Alphamab, 3D Medicines, and Simcere has been formally filed to the National Medical Products Administration of China (NMPA) on Nov. 16, with the indication applied for being microsatellite instability-high (MSI-H)/deficient mismatch repair (dMMR) advanced colorectal cancer (CRC), gastric cancer (GC) and other advanced solid tumors after standard treatment failure.

Nanobody for subcutaneous injection
As an IgG1-Fc fusion anti-PD-L1 nanobody independently developed by Alphamab, envafolimab has many advantages: unique antibody structure design, IgG1 subtype of Fc, and mutations of C221S, D265A and P331G that remove ADCC and other Fc effector effects; wide clinical indications, being a broad-spectrum oncology drug; subcutaneous injection that can be used for patients not suitable for intravenous infusion, being the world’s first subcutaneous anti-PD-L1 monoclonal antibody inhibitor; high thermal denaturation temperature, making it available for storage at room temperature, and high patient compliance.
Clinical basis for the BLA in China
This BLA for envafolimab injection in China is mainly based on the data of the phase 2 key clinical trial of envafolimab for MSI-H/dMMR advanced solid tumors (NCT03667170).
This clinical trial enrolled a total of 103 patients and used a single-arm, open-label design with the primary endpoint being the Objective Response Rate (ORR) confirmed by a blinded independent review committee (BIRC), with the MSI-H of CRC and GC pathologically confirmed by the center, and the dMMR of other tumors according to local pathological assessment results.
According to the clinical results, the ORR confirmed by the BIRC’s assessment in all the subjects (n = 103) was 42.7%, the ORR in CRC patients (n = 65) was 43.1%, the ORR in GC patients (n = 18) was 44.4%, and the ORR in other solid tumor patients (n = 20) was 40.0%. The median Duration of Overall Response (DOR) assessed by BIRC in all subjects was not reached, and the 12-month DOR rate was 92.2%. The median Progression-free Survival (PFS) was 11.1 months; the median Overall Survival (OS) was not reached and the 12-month OS rate was 74.6%.
Tripartite cooperation
3D Medicines, after signing a cooperation agreement with Alphamab early in Feb. 2016, has fast advanced the global clinical study of envafolimab and completed clinical trials involving a cumulative total of nearly 1,000 patients, proving the safety and effectiveness of envafolimab.
Alphamab, 3D Medicines, and Simcere reached a strategic cooperation agreement on Mar. 30, 2020, with the developer Alphamab responsible for the production and quality of the future product, 3D Medicines responsible for clinical development in the oncology area, and Simcere responsible for the exclusive commercial promotion of envafolimab in the Chinese mainland in the future. Want to buy medical products online? Pharmasources would be your best choice.
Global clinical layout
Clinical trials have so far been conducted for envafolimab injection in China, the U.S., and Japan, with clinical indications covering hepatocellular carcinoma, biliary tract cancer, HER2-positive breast cancer, fibrosarcoma, renal cell carcinoma, and other solid tumors. Among them, the clinical trial (NCT03478488) for biliary tract cancer is in phase 2. Envolizumab was granted an Orphan Drug Designation by the U.S. FDA in the middle of this year for the treatment of bile duct cancer.
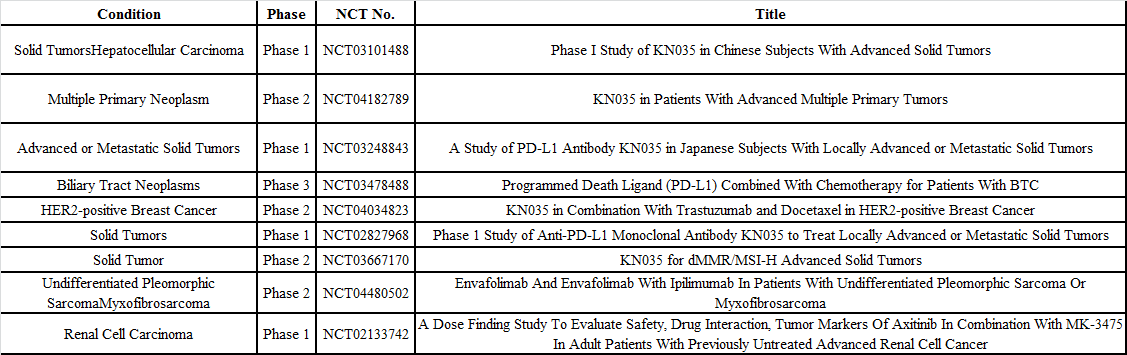
Two studies of envafolimab injection were presented at the 2020 ASCO (American Society of Clinical Oncology) Annual Meeting, with the data presented supporting envafolimab as a new, promising, and convenient treatment choice. It has long-lasting benefits for previously treated patients with advanced MSI-H/dMMR cancers and may become a first-line therapeutic regimen for advanced G/GEJ tumor.
Xiaoyaowan, a pharmaceutical industry practitioner, a word carrier in the We-media era focusing on changes of the pharma industry.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


