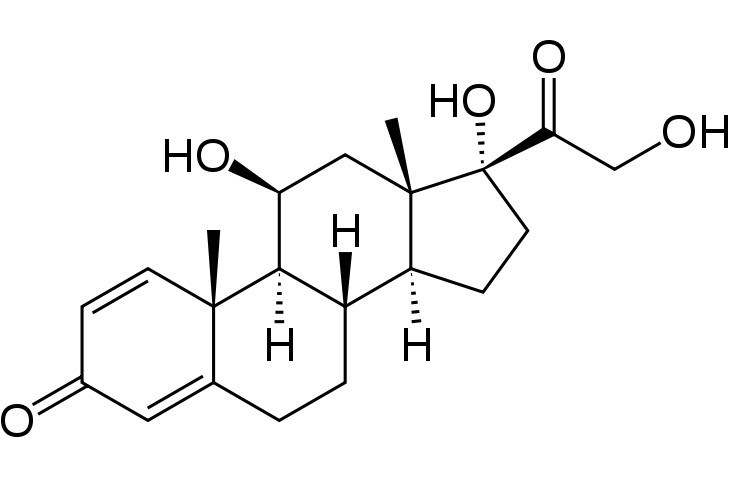
Amphastar Pharmaceuticals announced the U.S. Food and Drug Administration (FDA) approved the Company’s Abbreviated New Drug Application (ANDA) for Atropine Sulfate injection 0.1mg/mL in the 10 mL Luer-Jet® Prefilled Syringe System. For the past 40 years, the Company has sold and marketed the product under the “grandfather” exception (now termed “unapproved”) to the FDA’s “Prescription Drug Wrap-Up” program. Net revenues for the Company’s Atropine Sulfate injection for the year ended December 31, 2019 were $12.2 million.
“The FDA’s approval of our Atropine Sulfate product, a product often on the Agency’s Drug Shortage list, offers an opportunity to ensure quality products are produced at the highest standard and highlights Amphastar’s manufacturing capacity to fulfill such market needs,” Amphastar’s CEO and President, Dr. Jack Zhang, said.
The Company currently has four ANDAs filed with the FDA, which are targeting products with a market size of approximately $1.5 billion, three biosimilar products in development targeting products with a market size of approximately $13.0 billion, and nine generic products in development targeting products with a market size of approximately $12.0 billion. This market information is based on IQVIA data for the 12 months ended June 30, 2020. The Company is developing multiple proprietary pipeline products for injectable and intranasal dosage forms, including a new drug application for intranasal naloxone.
Amphastar’s Chinese subsidiary, ANP, currently has 14 Drug Master Files, or DMF, on file with the FDA and is developing several additional DMFs.





 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story































 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








