PharmaSources.comMarch 09, 2021
Tag: sterile APIs , jet mill , Pressure Filter Dryer
Most APIs are not heat-resistant, so non-final sterile APIs are the most common in real production. The production of non-final sterile APIs usually includes solvent based crystalline sterile APIs, freeze-dried sterile APIs and spray drying sterile APIs. The sterile APIs produced by solvent crystallization have the advantages of high quality, less impurities and good stability. Although the crystallization and drying processes are different, they are mainly transformed from non-sterile to sterile by sterilization and filtration, and keep aseptic during the whole drying and subsequent crushing, mixing and packing process after transformation. This article is a case study of a sterile solvent crystallization API systems. This production line is mainly used for the production of sterile drugs, to ensure consistent compliance of core process from solution preparation, crystallization, filtration and washing to final product pulverizing and dispensing with the design concept of product quality, environmental and personnel safety, in line with the development trend of high-end pharmaceutical production processes.
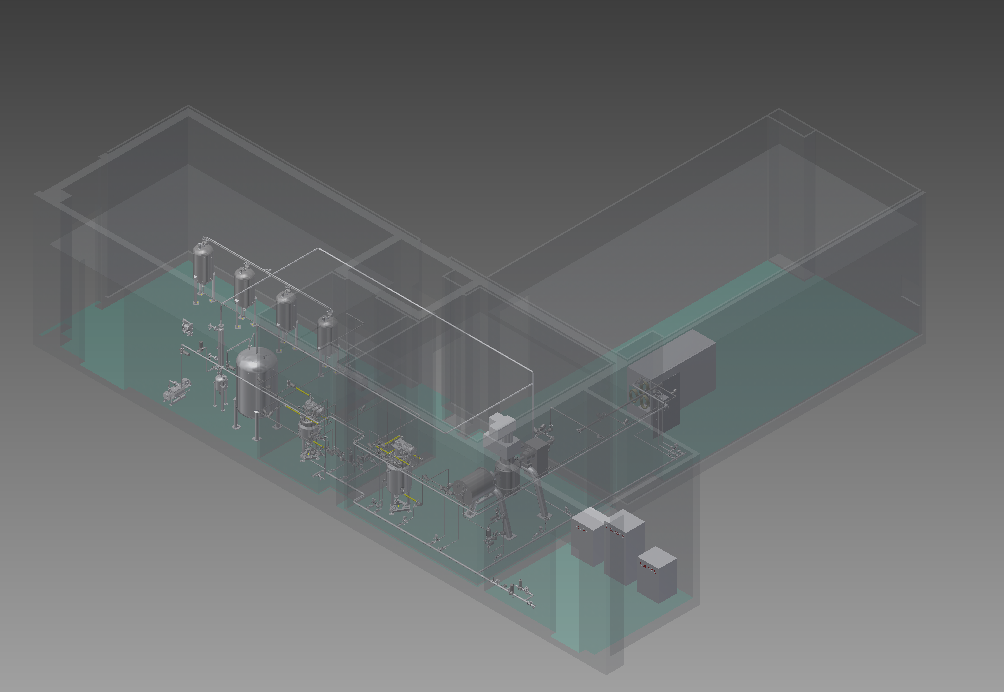
Fig.1Workshop Layout
Features of Workshop Layout
The production workshop is divided into Class A area (explosion-proof area) and Class C area (non-explosion-proof area) based on the fire risks of materials, which are partitioned by the explosion-proof wall.The integrated design has reduced the explosion-proof area. The modular design of the thermal medium and vacuum system are placed in the non-explosion-proof area to save upfront investment and daily operating costs.
The core process module and the solvent are separated by a wall only, which allows a short conveying distance of materials through the pipeline to avoid material loss due to pipeline residue.
The cleanroom environment is required to be controlled at the level C only, which has effectively reduced the cost of auxiliary facilities, personnel clothes changing, cleanroom environmental monitoring, daily energy consumption, etc.
If you want to know more about Sterile Solvent Crystallization API Systems, you could visit our website. As one of the hospital equipment companies, PharmaSources not only do medical equipment supply business but also order medical supplies online.
Process Module Design
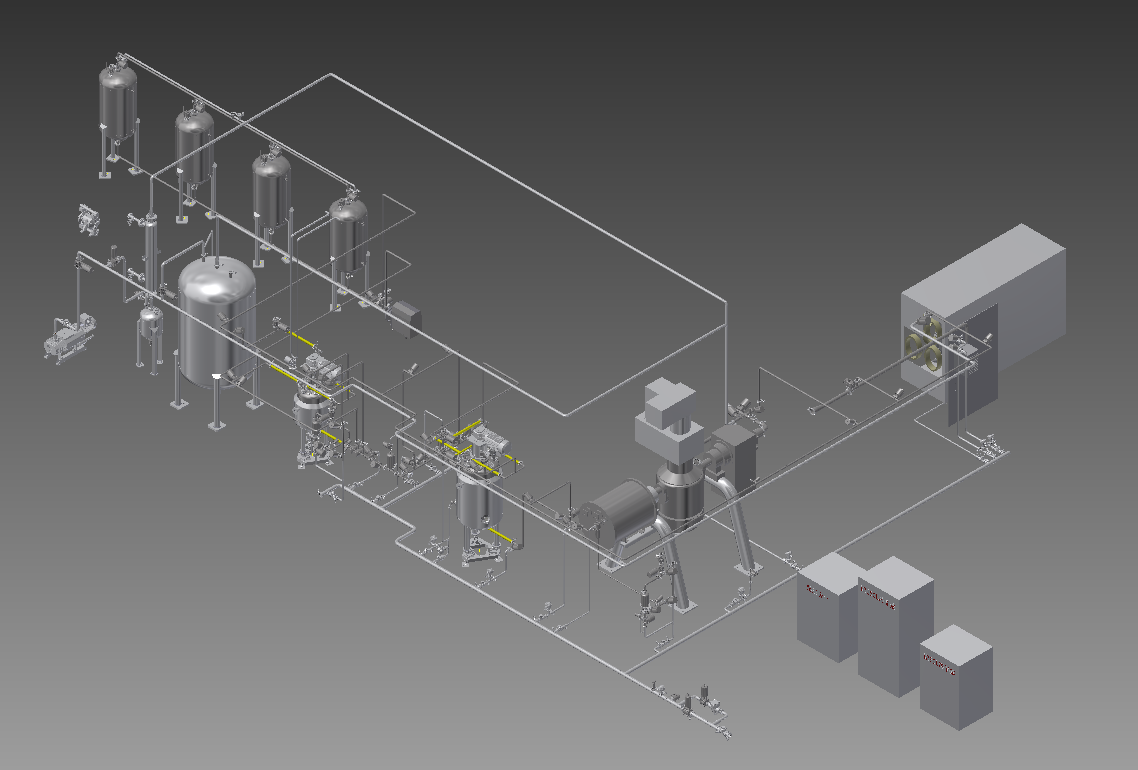
Fig. 2 Process Module Diagram
The reasonable layout, based on the upstream and downstream processes, is arranged to minimize the material conveying distance and avoid material loss during the transfer to the greatest extent.
The modular design of process pipelines, tanks, and pump sets has reduced the intensity of fieldwork. The process tubes are arranged on the pipe racks to ensure integrity and aesthetics. The equipment piping can be connected to the main pipe in the vicinity. Thus, the overall modular pipe arrangement is highly concise, beautiful and easy to maintain.
These factors were considered in the design process such as the orientation of public utilities, room return air and others. What’s more, overall consideration is given to the equipment module and room design, to avoid the risk of interference and winding after equipment entry and improve the integration of equipment and workshop.
Integrated design is adopted for the jet mill and isolator, which, on the one hand, can reduce the intermediate material transfer process and risks considering the sterility of discharging and dispensing; on the other hand, it also considers the sterile protection in the removal and assembly of some parts of jet mill during its cleaning and sterilization process.
Optimized process steps and rational equipment selection

Based on the respect for the process, ration equipment design is performed to reduce the equipment input cost and material exposure risk greatly. The traditional recrystallization process is as follows: crystallization in the crystalline tank - filtration and washing by centrifuge or suction filter - removing solids - drying. However, In our system, Pressure Filter Dryer (PFD), a new sterile solvent crystallization machine with characteristic structural, was ingeniously used to prevent the risks caused by traditional open operation or discontinuous transfer of units (such as the transfer among centrifuge, drying oven and pulverizer) effectively, reduce the input cost for the related process equipment (equivalent to the function of centrifuge and dryer), and avoid the personnel safety hazard due to material exposure or material loss due to the multi-step and multi-device transfer.
In the PFD model selection, Tilting PFD is selected for this project. Under the same conditions of materials and solvents, the discharge rate is 10% higher than the traditional PFD, and the drying time is shortened by 30%, which has effectively improved the production efficiency and yield, helping customers save production and time costs.
CPhI China 2020 To Be Postponed to Dec. 16-18th,
Register as Visitor to the 20th Edition of CPhI China!
-----------------------------------------------------------------------
Editor's Note:
To become a freelance writer of PharmaSources.com,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@imsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


