Lin ZhangNovember 12, 2020
Tag: CRO , drug development
Today, contract research organizations (CROs) provide crucial services for almost any point along the drug pipeline.
What are CROs?
Contract research organizations (CROs) are outsourcing firms that undertake some of the more precise and focused R&D functions for the pharmaceutical, biotechnology, medical device industries, and also serve government institutions, foundations, and universities. Their specialization in certain aspects of the development process or specific therapeutic fields underscores expertise in those areas. They tend to be more mistake-proof, consisting of highly experienced professionals, offering preclinical, clinical, regulatory activities, and other research support services for drug development and commercialization. CROs are comprised of classes of unique technology, full-service, multinational, publicly-traded firms. Midsized CROs have the capability to act as site management contractors, which provide the clinical team, physician investigators, and patient support for clinical trials at the site. CROs can oversee and complete an administrative function in clinical trials or affiliate with an established project. Their main function is to close the “time-to-marketing” gap through effective application of innovation, engaging in a full spectrum service for the small biotech companies’ clinical trial activities. Therefore, the relationship is business, and the contract is for deliverables. (Table 1). (1)
Table 1: Examples of CRO Services
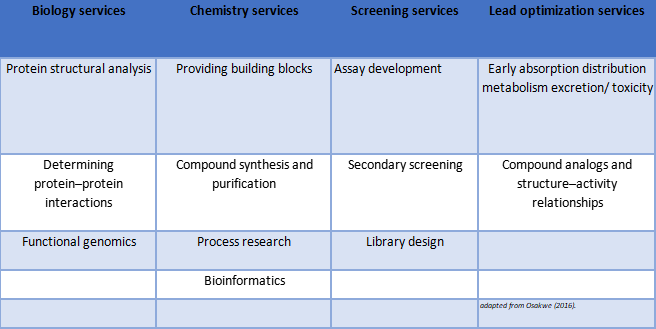
Moreover, a contract research organization (CRO) is a hired agent who has corresponding knowledge, technology and experience to conduct and complete tasks for a sponsor.
Types of CROs
There are different types of CROs, depending on the specific services they offer. Typically, CROs are segmented in discovery, preclinical, clinical, laboratory services, regulatory approval and data management. It is important to note that an individual CRO may specialize in one or more areas mentioned. Medium-sized CROs and larger may offer services ranging from discovery through clinical-stage trials all in one company.
Discovery
Drug discovery is the largest segment by service type and accounts for approximately 33%, of the total CRO market. (2) CROs specializing in drug discovery contain well-equipped laboratories focused on the discovery and preformulation/formulation sectors of the pipeline. Such companies tend to employ primarily chemists, scientists, and regulatory professionals and are focused on working with customers to quickly and efficiently move through the early steps of the drug pipeline.
Preclinical
CROs specialized in preclinical stages focus their efforts to studying the stability and efficacy of an API to the manufacturing of contracted drug products. Such companies will highlight services i.e. stability studies, a functioning vivarium, or pilot plants. They will have the systems in place to perform long-term multi-year experiments per contracted API.
Clinical
Clinical trials are complex, requiring study participants such as patients/volunteers, investigators/physicians, sponsors, nurses, quality oversight, and case management. CROs specializing in clinical phase have the personnel to set-up, site visit, run and document the entire process. Due to the high level of skill and regulation required for clinical-stage trials, it is not uncommon for CROs with a specialization in this area to not offer many other services in an effort to keep operating costs minimized. The fundamental theme of clinical phase is to apply what is revealed in preclinical experimentation and to bring the resulting safety and efficacy to clinics.
Laboratory Services
We know that laboratory equipment is expensive and staffing experts who can not only run the instruments but interpret the data can cost more than the equipment in the long run. While Big Pharma can usually afford to staff multiple labs full of state-of-the-art equipment, many biotechs and start-ups do not have the capital resources necessary. Thus, many CROs have chosen to specialize in laboratory services. While keeping a handful of senior-level scientists on hand, they can employ teams of technicians to collect data on samples submitted by customers. In the cGxP space (cGxP stands for FDA compliance Current Good X Practice (X can mean: Clinical, Laboratory, Manufacturing, Pharmaceutical), this results in data along with a Certificate of Analysis signed off by the scientist in charge.
Advantages of Hiring CROs
CROs can add considerable value to the processes of drug development and clinical trial, which provides a cost-saving solution for clients. CROs also serves as a regulatory point of control, owning the processes which are contracted to them i.e. FDA regulatory audits which is a time consuming and can be costly. Having the CRO take ownership of the regulatory process frees the contracting company’s time and budget, along with requiring less personnel who are regulatory experts to be on staff. CROs also generally employ their own QA/QC departments/auditors, giving the contracting company an already integrated check on the data before it ever leaves the CRO. While companies who source to CROs will want to have a Principal or Senior Scientist overseeing the project, utilizing a CRO will free up a significant portion of their workforce to focus on other important projects.

Potential Markets for CROs
Contract research organizations (CROs) represent a multibillion-dollar market that is firmly embedded in the contemporary clinical trial process.
The global CRO industry was estimated at $31.6 billion in 2018 and was expected to reach $45.2 billion by 2022, according to a report. (2-3)Over the past decades, the reach of CROs has extended to service all phases of drug development and clinical trials in an increasingly global research environment.
The clinical research landscape, which had been largely confined to the US and Western Europe, first expanded to Eastern Europe and Latin America and in the late 1990s and then further expanded into Japan, China and other Asia-Pacific (APAC) countries thereafter. The true globalization of clinical trials facilitated the substantial expansion of CROs into these regions. In addition to expanding their global footprint, this expansion also afforded the CROs to opportunity to tap into a larger pool of highly qualified resources to support their clinical and laboratory businesses in those regions.(4)
In today’s market, especially with the global push for a vaccine as well as therapeutics to battle the COVID-19 pandemic, CROs will provide a crucial function especially in the role of clinical trials. In an interview with Contract Pharma in Oct. 2019, Randy Marchbanks highlighted the role of CROs in the near-term, stating:
The need to identify increasingly difficult to find subjects for clinical trials influenced the biopharmaceutical industry and CROs to expand their geographic interest and footprint to new global markets over the past 20 years. Coincident with that, government agencies, regulatory bodies, and ethics professionals around the world initiated the processes of harmonization, standardization, and the governance of clinical trials globally. This harmonization allowed biopharma companies to design and execute global trials in support of marketing applications in several countries, negating the need for local trials to support marketing of new products.
Conclusion
It has become increasingly obvious that CROs play pivotal roles and provide crucial services in each phase of discovery, developing product to market through in vitro, in vivo, and ex vivo testing during preclinical experimentations and clinical studies along the drug pipeline.
CROs can provide a valuable interface between the contracting company and regulatory agencies as the CRO takes responsibility for all of the portions of the pipeline for which they provide service for a particular project. This is extremely advantageous for smaller biotech and startup companies who might not have as much experience dealing with the regulatory aspects of drug development.
Therefore, the CRO industry’s biggest challenge and greatest goal, is addressing the next generation drug development landscape by re-evaluating how trials are conducted and leveraging data in new ways to drive efficiency into the process of drug development.
References
1. Social Aspects of Drug Discovery, Development and Commercialization. (2016)
2. Credevo Articles. https://credevo.com/articles/2019/08/16/how-to-choose-a-cro-small-mid-size-pharma-companies/ (2019)
3. Cancer, 2016 May 15;122(10):1476-82.
4. Contract Pharma. https://www.contractpharma.com/contents/view_online-exclusives/2019-10-28/contract-pharmas-20th-anniversary-series-cro-perspective/ (2019)
About the Author:
Lin Zhang, M.D., senior director of a health care industry company in the United States. With the experience in clinical medicine, biotechnology, health industry and other fields, he is responsible for the research and development of plant medicine, functional food and health products. He was a clinician and worked for the National Cancer Institute, FDA and the National Cancer Center of Japan for many years.
-----------------------------------------------------------------------
Editor's Note:
To become a freelance writer of PharmaSources.com,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@imsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


