PharmaSources/XiaoyaowanAugust 21, 2020
New update:
It has been reported by the CPhI.CN on Aug. 18th that the COVID-19 inactivated vaccine production workshop of the Beijing Institute of Biological Products of Sinopharm Group has passed the joint biosafety inspection organized by the relevant national departments and has the conditions for use. With the price of two vaccinations being less than 1000 RMB, the protection could reach 100% according to the president of Sinopharm Group. The inactivated vaccine is expected to be marketed by the end of 2020, with the annual capacity of over 200 million.
The CDE issued five documents including the Technical Guidelines for the Research and Development of Prophylactic Vaccines against COVID-19 (Trial) on Aug. 14, and among them, the Guidelines for the Clinical Evaluation of Prophylactic Vaccines against COVID-19 (Trial) (“Guidelines”) gives the evaluation criteria for the marketing of COVID-19 vaccines in terms of clinical needs, safety, effectiveness, marketing evaluation, and overseas clinical trial data, etc. Those COVID-19 vaccine R&D guidelines are issued to guarantee quality and speed up the marketing of COVID-19 vaccines in China.

Criteria for COVID‑19 vaccine candidates given in the Guidelines
Target population
COVID‑19 vaccine candidates should preferably apply to all age groups, including pregnant and lactating women and should at least apply to adults, including the elderly.
Safety
The adverse reactions should be mild with short duration, and there should be no serious adverse reactions or the serious adverse reaction rate should be extremely low. COVID-19 vaccines shall not have ADE risk.
Protective efficacy
In placebo-controlled trials, the protective efficacy in the target population should preferably reach more than 70% (point estimate) and at least 50% (point estimate), with the lower limit of the 95% confidence interval of not less than 30%. Vaccines should preferably provide protection for 1 year or more and at least 6 months.
The immunization procedure allowed to be changed during clinical trials
It is permitted to enter phase III clinical trials before the most appropriate immunization procedure and dose are determined, and changes to the immunization procedure (such as increasing the number of vaccination doses) may be considered during phase III clinical trials or the immunization procedure may be optimized after marketing. Various immunization procedures and vaccination routes are acceptable as long as they can provide long-term protection. Vaccines that can rapidly provide protection with fewer doses and shorter vaccination intervals and those that have convenient administration routes will have more advantages during epidemic outbreaks.
Conditional marketing applications
If the interim analysis results of a phase III clinical trial show definite and acceptable protective efficacy, but the clinical trial has not been completed and the results are not robust enough to meet the criteria for early termination of the trial, upon a benefit-risk assessment, the data may be used to apply for conditional marketing approval, and the clinical trial shall continue to be completed.
Data basis to support marketing in China
The data used for vaccine evaluation, regardless of whether they are from clinical trials in China or outside China, may be considered as an important basis for supporting the marketing of vaccines in China if the data source, data quality, and trial results are assessed to meet the requirements.
Criteria for COVID-19 vaccines issued by international counterparts of the CDE
The WHO and U.S. FDA already issued standard guidelines for the development or evaluation of COVID-19 vaccines before the CDE issued the above five guideline documents.
The WHO issued the Target Product Profiles for COVID-19 Vaccines on Apr. 9, which lists the WHO’s preferred and minimal criteria for multiple indicators of COVID-19 vaccine products.
The U.S. FDA published the Development and Licensure of Vaccines to Prevent COVID-19 on July 1 to share its current thinking on many issues in the development and approval of COVID-19 vaccines.
The CDE’s issuance of a series of guideline documents on COVID-19 vaccines in China this time will promote China’s COVID-19 vaccine R&D and evaluation systems or criteria to be in line with international criteria to better contribute to humanity’s fight against COVID-19.
Advantages and disadvantages of different technical routes for COVID-19 vaccines
From the perspective of the technical routes for COVID-19 vaccine development in the world, nucleic acid vaccines and recombinant vaccines account for about half, and each technical route has its advantages and disadvantages: inactivated virus vaccines have the simplest process, however, their immunizing potency is relatively low; adenovirus vaccines provide good tolerance, however, the anti-adenovirus antibodies in some people can cause the vaccines to fail; mRNA vaccines can be produced fastest, however, they are easily degraded.
Seven COVID-19 vaccines in the world that have been published clinical trial data
The clinical data have so far been successively disclosed for seven COVID-19 vaccines in the world. According to the existing clinical trial data, the COVID-19 vaccines in development have demonstrated humoral and cellular immunity and the data support the conduct of phase II/III clinical trials.
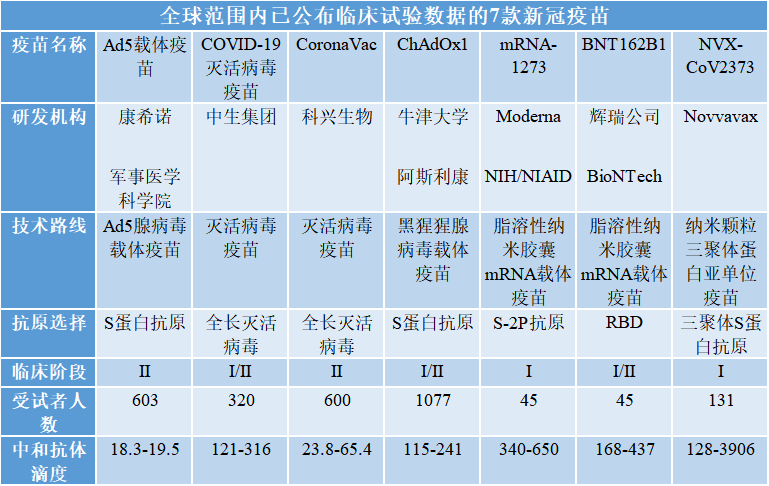
Source: Organized according to public data
Almost all infectious disease vaccines marketed in the world at present achieve body protection through the induction of neutralizing antibodies, and neutralizing antibody titer can reflect the strength of protection to a certain extent. According to the data in the above table alone, CNBG’s COVID-19 inactivated virus vaccine is the best vaccine developed in China in inducing serum neutralizing antibody activity and is as good as the vaccines from Pfizer, AstraZeneca, and Moderna; this vaccine had mild adverse reactions, and the phase III clinical trial has begun.
It should be noted that there is no global standard for COVID-19 vaccines at present. There are various technical routes, different entities use their respective standard system to develop COVID-19 vaccines, and different institutions use different neutralization test methods, units and statistical methods; even with the same method, the results can vary greatly depending on the strain used. Therefore, horizontal comparisons of absolute values do not provide much guidance.
Furthermore, the relationship between antibody titer and clinical protection rate and the mechanism of humoral and cellular immunity for the prevention of COVID-19 attack have not been established, therefore, the effectiveness of the vaccines cannot be determined according to the existing clinical data, regardless of the technical route. More answers about COVID-19 vaccines may only be revealed from the results of phase III clinical trials.
Xiaoyaowan, a pharmaceutical industry practitioner, a word carrier in the We-media era focusing on changes of the pharma industry.
-----------------------------------------------------------------------
Editor's Note:
To become a freelance writer of PharmaSources.com,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@imsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


