PharmaSources/LaoxueSeptember 23, 2020
Tag: NMPA , Chinese Drug Regulator , Japanese Enterprise
The Japanese pharmaceutical enterprise: Sanyo Chemical Laboratories Co., Ltd.’s refusal of the NMPA’s inspection was disclosed and exposed by the NMPA on July 31. The detailed written report is not disclosed, however, the NMPA mentioned on its website: “As the enterprise expressed in writing its refusal of the onsite inspection, the enterprise was deemed as refusing the inspection according to the Provisions on the Administration of Overseas Inspection of Drugs and Medical Devices.”

Source: NMPA
My first reaction to this news was, “Will there be a mandatory recall?” Then, I came to know that the product involved was an API upon inquiry. Then I started to worry whether it would result in an interruption of supply to Chinese pharmaceutical enterprises. We have been aware of how arrogant API enterprises could be, for example, an enterprise dared to directly throw away the USB flash drive of the NMPA’s inspection team, which was something other types of pharmaceutical enterprises would never dare to do. And we know how far China will punish such kind of deed: even up to RMB100 million is possible.
Then, why this Japanese pharmaceutical enterprise did it? With this question, I searched the related data and discovered that only this enterprise sells imported methoxyphenamine hydrochloride, i.e., only it exports this drug to China.
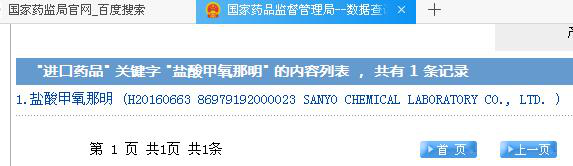
Source: NMPA
Then, how about the Chinese pharmaceutical enterprises? The results seem surprising. There is also only one Chinese API pharma companies, and there are ten formulation enterprises.
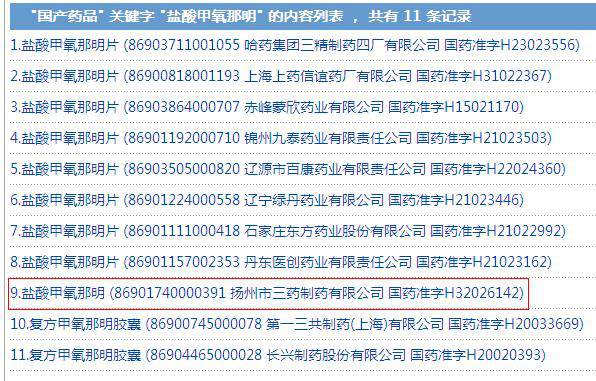
Source: NMPA
Here is the question: is the number of enterprises producing that API really this small or does the NMPA not timely update the data? I choose to believe the former. Please note that the following descriptions are based on data disclosed by the NMPA. If there is any omission, please urge the drug administration to update the data. Any comments are welcome.
There are two possible reasons for this Japanese enterprise’s daring to refuse the inspection in writing. Firstly, they do not take this market seriously because the export volume is too small. Is this possible? Definitely. In particular, the export and transportation costs have increased greatly during this pandemic, therefore, the enterprise might choose to give up. If this was the case, it would indirectly show the absolute market share of Yangzhou No. 3 Pharmaceutical in methoxyphenamine hydrochloride variety. Can we check which enterprises use the API of this Japanese enterprise and which enterprises use that of Yangzhou No. 3 Pharmaceutical? Yes, but only among those listed.
However, as the saying goes, a foxy person has more than one hideout. Pharmaceutical enterprises are likely to list both enterprises as API suppliers and they would even choose three API suppliers if there was another. This is something right to do because, apart from anything else, they will have to wait for production halt if they do not choose Yangzhou No. 3 Pharmaceutical as a backup. The leaders of enterprises not choosing a backup can scold the registration/R&D/QA personnel, like, “How did you manage risks? Have you never considered the situation of suppliers quitting?” And if the personnel answer, “I never thought enterprises dared to refuse inspections. It was not our responsibility”, the leaders can continue to scold, “You could not even foresee that, so, what is your use? Isn’t a plan B something we should always prepare?”
Another possible reason is that the API of this Japanese enterprise accounts for a very large share in China and even reaches a monopoly state, which gave the enterprise the courage to refuse the inspection, just like the following circumstance: there is only a restaurant in a hundred-mile radius, and you want to inspect the kitchen cleanliness after ordering dishes, but the lobby manager may directly ask you to leave, “Eat it or not.” You have no other choice but to accept it. If it was this case, the refusal of inspection would badly hurt downstream producers. Without the API, not knowing when the import will resume, and not daring to increase prices, they have to use all their inventory and then stop production.
However, it’s a pity that I did not find the marketing data of methoxyphenamine hydrochloride or its comparison with other bronchial asthma drugs, therefore, I could not answer the question with certainty. But the normal development of the pharmaceutical industry will certainly be affected if any API enterprise has any dominance in any variety. This is a lopsided pattern not matter for the public or pharmaceutical industry. A person fickle in love is a scumbag, but a pharmaceutical enterprise choosing more API suppliers as backups is one with high awareness of risk control. It is hoped that pharmaceutical enterprises could learn a lesson from the case to improve their awkward situation in relation to APIs.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


