PharmaSources/DopineDecember 24, 2020
Tag: Chinese new drugs , production applications , Targets , constituent type
According to the Insight database, the CDE generated 50 marketing application acceptance numbers for Chinese new drugs in 2020 H1, involving 36 varieties. Seen from the constituent type, monospecific antibodies had the largest number (12), followed by chemical drugs (11), and there were two CAR T-cell products, among the Chinese new drugs applied for production in 2020 H1.
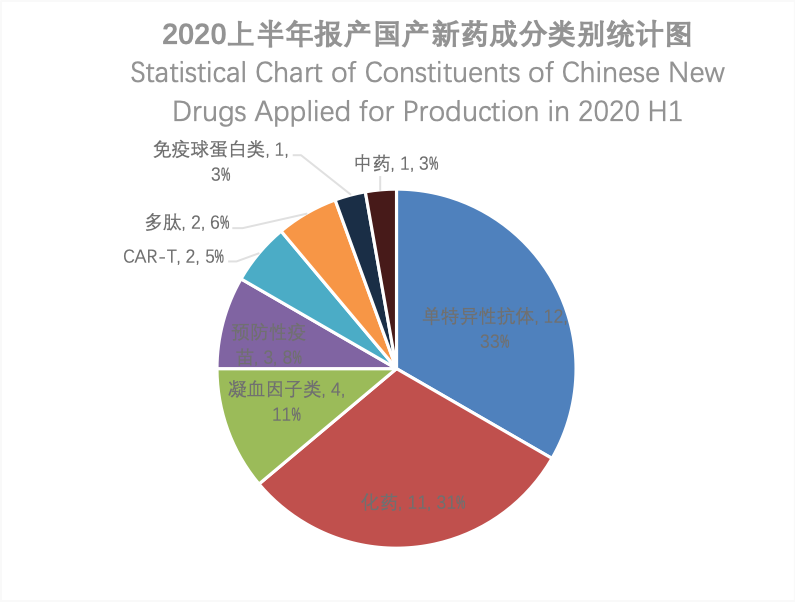
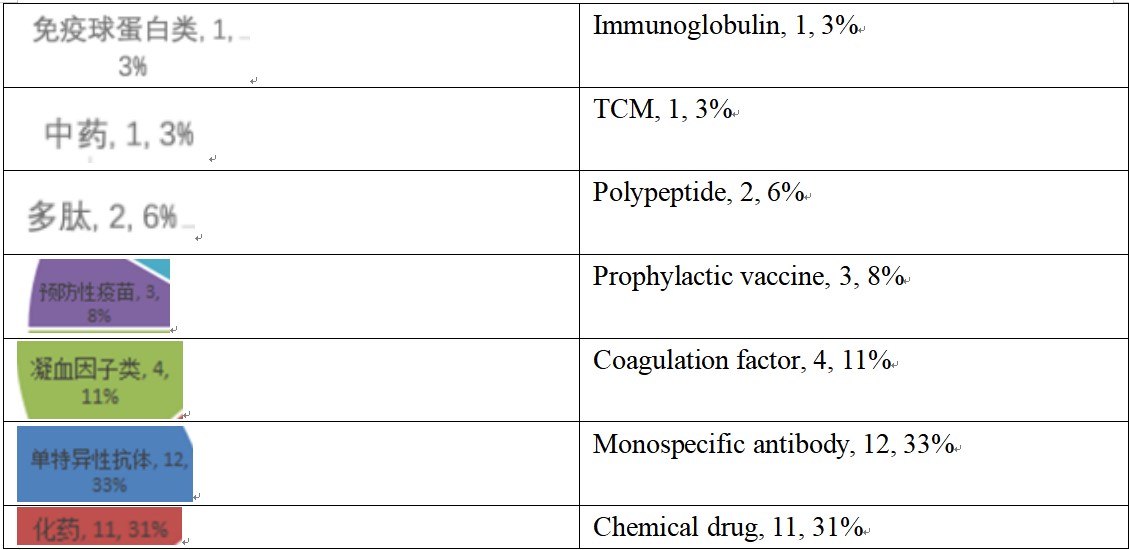
Calculated according to varieties, among the 11 Chinese new chemical drugs applied for production in 2020 H1, 7 were Class 1 new drugs, and 4 were modified new drugs. From the perspective of therapeutic areas, almost half were oncology drugs, followed by antibiotics.
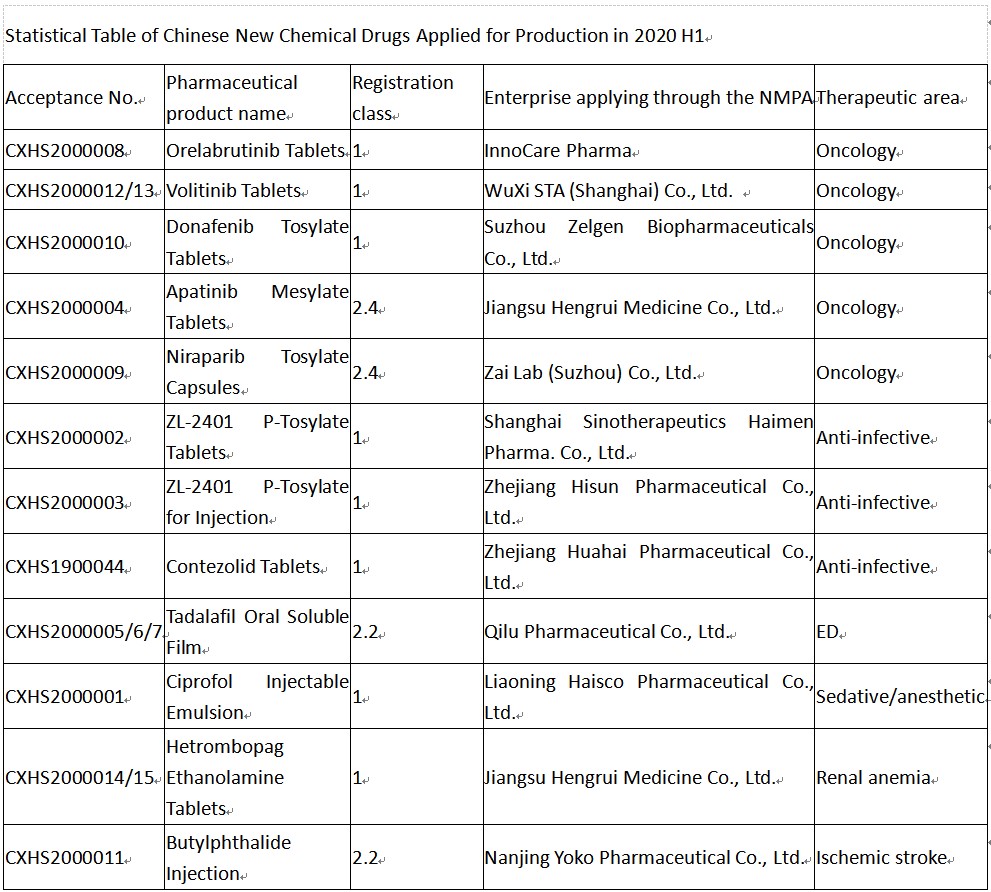
Among the 5 oncology drugs, only volitinib and donafenib were applied for production for the first time, and the other 3 were applied for new indications.
As a selective MET inhibitor developed by Hutchison MediPharma, volitinib was applied for the indication of NSCLC with mesenchymal-epithelial transition (MET) exon 14 skipping mutations and is expected to become the first Chinese-produced MET inhibitor approved.
Donafenib is an oral, multi-targeted inhibitor of multiple kinases developed by Zelgen Biopharmaceuticals as a small molecule oncology drug, which can inhibit the activity of multiple receptor tyrosine kinases such as VEGFR and PDGFR and also directly inhibit various Raf kinases and inhibit downstream Raf/MEK/ERK signal transduction pathway to inhibit tumor cell proliferation and tumor angiogenesis. The indication applied for this time was advanced (inoperable or metastatic) hepatocellular carcinoma.
As a selective BTK inhibitor independently developed by InnoCare Pharma’s team, the first NDA was filed for orelabrutinib in November last year for the treatment of relapsed/refractory chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). The indication applied for this time was relapsed/refractory mantle cell lymphoma (MCL).
Apatinib mesylate is an oral small molecule anti-angiogenesis inhibitor approved for the treatment of patients with advanced gastric adenocarcinoma or gastric and gastroesophageal junction adenocarcinoma who progressed or relapsed after at least 2 prior systematic chemotherapy regimens. The indication applied for this time was liver cancer.
Niraparib is an oral small molecule inhibitor of PARP 1/2 developed by TESARO and was approved in China in Dec. 2019 for the maintenance treatment of recurrent ovarian cancer. The indication applied for this time was its second indication, which is the first-line maintenance treatment of ovarian cancer.
2 new antibiotics applied for production for the first time to be used against drug-resistant bacterial infections.
Contezolid belongs to oxazolidinone antibacterials and is used to treat infections caused by drug-resistant bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE), which will provide doctors and patients with a safer and better-tolerated treatment choice than existing oxazolidinone drugs.
ZL-2401 p-tosylate, i.e., omadacycline, is a new tetracycline designed to overcome tetracycline resistance and has broad-spectrum antibacterial activity, including against gram-positive bacteria, gram-negative bacteria, atypical pathogens, and multiple drug-resistant strains. The indication applied for production was community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSI).
Furthermore, the other 4 therapeutic drugs should not be taken lightly. Hetrombopag ethanolamine is an oral small-molecule non-peptide thrombopoietin receptor (TPO-R) agonist independently developed by Hengrui, which is used to treat thrombocytopenia. Ciprofol Injectable Emulsion is a brand-new intravenous anesthetic developed by Haisco with independent intellectual property rights, which will be indicated for the induction of general anesthesia in surgery, sedation/anesthesia in endoscopic diagnosis and treatment, and ICU sedation, etc.; its indication for gastrointestinal endoscopic diagnosis and treatment and/or anesthesia has been accepted by the CDE in Aug. 2019, and the indication applied for this time was the induction of general anesthesia. Nanjing Yoko’s Butylphthalide Injection is a modified new drug corresponding to CSPC’s Enbipu (Butylphthalide and Sodium Chloride Injection), which is used to improve the neurologic impairment of patients with acute ischemic stroke. Qilu Pharmaceutical’s Tadalafil Oral Soluble Film is also a modified new drug. It is worth mentioning that no oral soluble film has been approved in China.
The CDE generated 33 marketing application acceptance numbers for Chinese biological pharma products in 2020 H1, including 5 for prophylactic ones, and 28 for therapeutic ones.
Among the acceptance numbers generated for therapeutic biological products, except those for biosimilars such as adalimumab, bevacizumab and infliximab biosimilars and non-original biological products such as recombinant insulin lispro (glargine), thrombin, and immunoglobulin, there were only 12 marketing application acceptance numbers of original biological products (only involving 7 varieties), and the targets of the 7 varieties are mainly PD-1 and CD19.
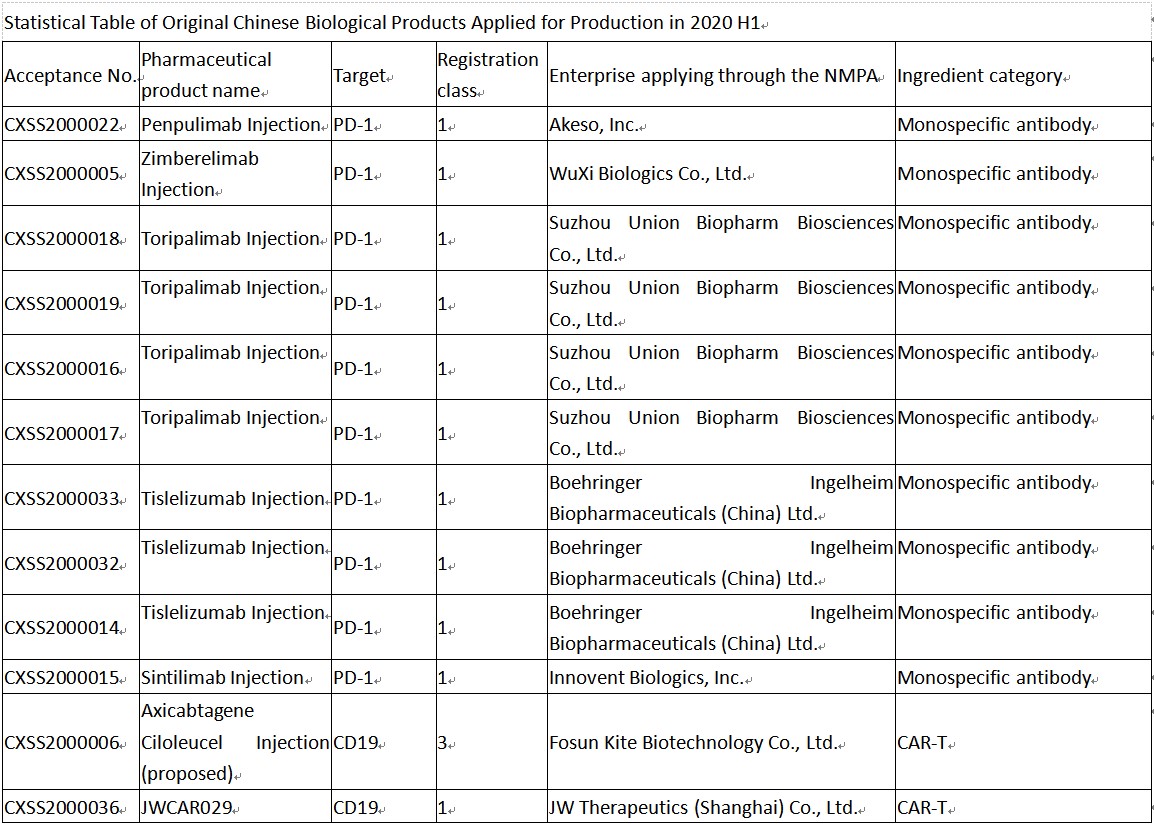
Chinese anti-PD-1 monoclonal antibodies have attracted wide attention in 2020 H1, with new indications applied for one after another. In 2020 H1 alone, sintilimab was applied for 1 indication, toripalimab was applied for 2 new indications, tislelizumab was applied for 3 indications, and Hengrui’s camrelizumab was not applied for new indications, however, it was successively approved for 3 new indications.
Furthermore, two new drugs are expected to enter the Chinese anti-PD-1 monoclonal antibody market, i.e., Akeso’s penpulimab and WuXi Biologics’ zimberelimab. Penpulimab (AK105, anti-PD-1 monoclonal antibody) is developed and commercialized by the joint venture established by Akeso and Chiatai Tianqing, an enterprise of Sino Biopharmaceutical, which is engineered to eliminate Fc receptor and complement-mediated effect through Fc region mutations. Compared with anti-PD-1 antibodies marketed overseas, penpulimab has slower antigen binding and unbinding rates, which enables it to block PD-1 pathway activity more effectively and maintain stronger antitumor activity of T cells. The first indication applied for penpulimab is relapsed or refractory classical Hodgkin’s lymphoma (cHL). Zimberelimab is a recombinant fully human anti-PD-1 monoclonal antibody developed by WuXi Biologics as commissioned by Gloria Biosciences, with the first indication applied for being the treatment of patients with relapsed or refractory classical Hodgkin’s lymphoma (r/r cHL) who received two lines of therapy.
Besides the anti-PD-1 monoclonal antibodies that have been quite hot in recent years, two CAR T-cell products were also applied for production in 2020 H1, i.e., Fosun Kite’s Axicabtagene Ciloleucel Injection and JW Therapeutics’ JWCAR029.
Axicabtagene ciloleucel is an autologous CD19-directed CAR T-cell therapy manufactured in China with the technology of YESCARTA® (axicabtagene ciloleucel) transferred from Kite Pharma, a Gilead company. It is worth mentioning that axicabtagene ciloleucel is the first CAR T-cell product applied for production in China, with the indication applied for being the treatment of adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, primary mediastinal B-cell lymphoma (PMBCL), high-grade B-cell lymphoma, and DLBCL arising from follicular lymphoma.
JWCAR029 is a CAR T-cell product independently developed by JW Therapeutics based on JCAR017 of Juno Therapeutics and the second CAR T-cell product applied for production in China, with the indication applied for being relapsed or refractory B-cell non-Hodgkin’s lymphoma.
-----------------------------------------------------------------------
Editor's Note:
To become a freelance writer of PharmaSources.com,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@imsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


