The hottest event of the week was the official kick-off of the centralized procurement of the third batch of pharmaceutical products organized in China, with 86 specifications started to be acquired the basic information. Regarding other aspects, no new drugs other than Humanwell’s Class 1 new drug remimazolam besylate were approved for marketing. Now, let’s review the hot events of the pharmaceutical industry this week. The news involved 6 sections: approval, R&D, performance, centralized procurement, listing, and transactions that happened during July 20-24, including 24 pieces.
Approval
NMPA
1. Humanwell’s Class 1 new drug “Remimazolam Besylate for Injection” was approved by the NMPA for marketing on July 20 for sedation during treatment or diagnosis operation. As an ultra-short-acting benzodiazepine sedative for intravenous injection developed by Paion, Humanwell obtained the license in 2012 to develop remimazolam in China through an agreement involving EUR3 million upfront payments plus subsequent share from sales.
2. Kelun’s Class 4 generic drug “Vardenafil Hydrochloride Tablets” entered the administrative approval stage on July 20, which is expected to be approved soon to become the first generic of the variety in China. Developed by Bayer, vardenafil, together with tadalafil and sildenafil, is an inhibitor of PDE5, which is used to solve the erectile dysfunction of males. The original drug was approved by the FDA in Aug. 2003 and entered China in June 2004, with a trade name of Levitra.
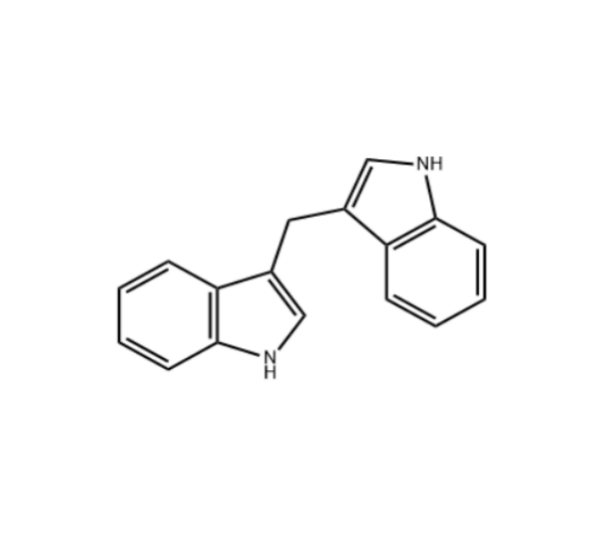
3. Zai Lab and Deciphera Pharmaceuticals announced on July 20 that the NMPA accepted the NDA of ripretinib for the treatment of adult patients with advanced gastrointestinal stromal tumor (GIST) who have received prior treatment with 3 or more kinase inhibitors, including imatinib. Ripretinib has recently been approved for marketing in the U.S. and other places.
4. Qilu Pharmaceutical’s Class 4 generic drug “Aprepitant Capsules” was approved by the NMPA for marketing on July 20 and deemed as passing the consistency evaluation to become the first generic of its variety in China. As a highly selective antagonist of substance P / neurokinin-1 (NK1) receptor, aprepitant is used for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of highly emetogenic cancer chemotherapy (HEC).
5. CSPC’s “Imatinib Mesylate Tablets” passed the consistency evaluation in China on July 20. Imatinib has been included in the NRDL (National Reimbursement Drug List of China) Category B List, with manufacturers in China including Novartis, the developer, Hansoh, Chiatai Tianqing, and CSPC. The drugs of Hansoh and Chiatai Tianqing have passed the consistency evaluation and won the bid with prices of RMB10.38/tablet and RMB9.77/capsule in the procurement with target quantity in the alliance regions of China.
6. Hangzhou Minsheng Pharmaceutical’s Class 3 generic drug “Ibuprofen Injection” was approved by the NMPA for marketing on July 20, making it the third enterprise with the pharmaceutical product deemed as passing the consistency evaluation in China.
7. “Acarbose Tablets”, a Class 4 generic drug, applied for by Hisun and CSPC was separately approved by the NMPA on July 20, which was deemed as passing the consistency evaluation. Before this, Acarbose Tablets of Huadong Medicine and Winsunny Pharmaceutical and Acarbose Capsules of Luye Pharma have been approved and deemed as passing the consistency evaluation.
8. Qilu Pharmaceutical announced on July 21 that it received the approval issued by the NMPA for the pharmaceutical product supplementary application of “Isosorbide Mononitrate Sustained-release Tablets” (30mg, 40mg), making it the first enterprise in passing the consistency evaluation of the product in China.
9. MaxiNovel Pharmaceuticals announced on July 22 that it applied to the NMPA for designating its drug MAX-40279 in development as a breakthrough therapy for treating wild-type FLT3 acute myelocytic leukemia (AML). FLT3 is a key target in treating AML, and the FLT3 inhibitor approved in the world only includes Astellas’ gilteritinib that was approved by the FDA in 2018 and has been filed the NDA in China.
10. Hengrui announced on July 22 that its HER2 targeted ADC: SHR-A1811 for injection was approved for a clinical trial to treat advanced HER2-expressing or HER2-mutant advanced solid tumors. Accumulatively about RMB41.60 million R&D expenses have been invested in the product.
11. Joincare announced on July 23 that its Shenzhen Taitai Pharmaceutical Industry’s “Budesonide Suspension for Inhalation” (2ml:0.5mg) was approved by the NMPA, making it the second enterprise approved for the variety in China and the first enterprise approved for the specification of the variety in China. Joincare has accumulatively invested about RMB24.5607 million R&D expenses in the related project.
Related News:
Pharmaceutical News of the Week (July. 20th-July. 24th) | PharmaSources.com - Updates on R&D
About the Author:
Caicai, a Master of Pharmacy from Shanghai Jiaotong University, used to work in the Institute of Science and Technical Information. Currently as a practitioner in the drug surveillance system, she is good at interpreting industry regulations, pharmaceutical research developments, etc.
-----------------------------------------------------------------------
Editor's Note:
To become a freelance writer of PharmaSources.com,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@imsinoexpo.com.





 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story






























 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








