PharmaSources/MilingJuly 22, 2020
Tag: DPP-4 , diabetes drugs , linagliptin
Guangdong HEC Pharmaceutical’s Class 4 generic drug: Linagliptin Tablets and Nanjing Sanhome Pharmaceutical’s Class 4 generic drug: Vildagliptin Tablets were both approved in China on July 13, which will bring huge changes to the market pattern of DPP-4 inhibitors in China and receive significant attention. Let’s now check them.
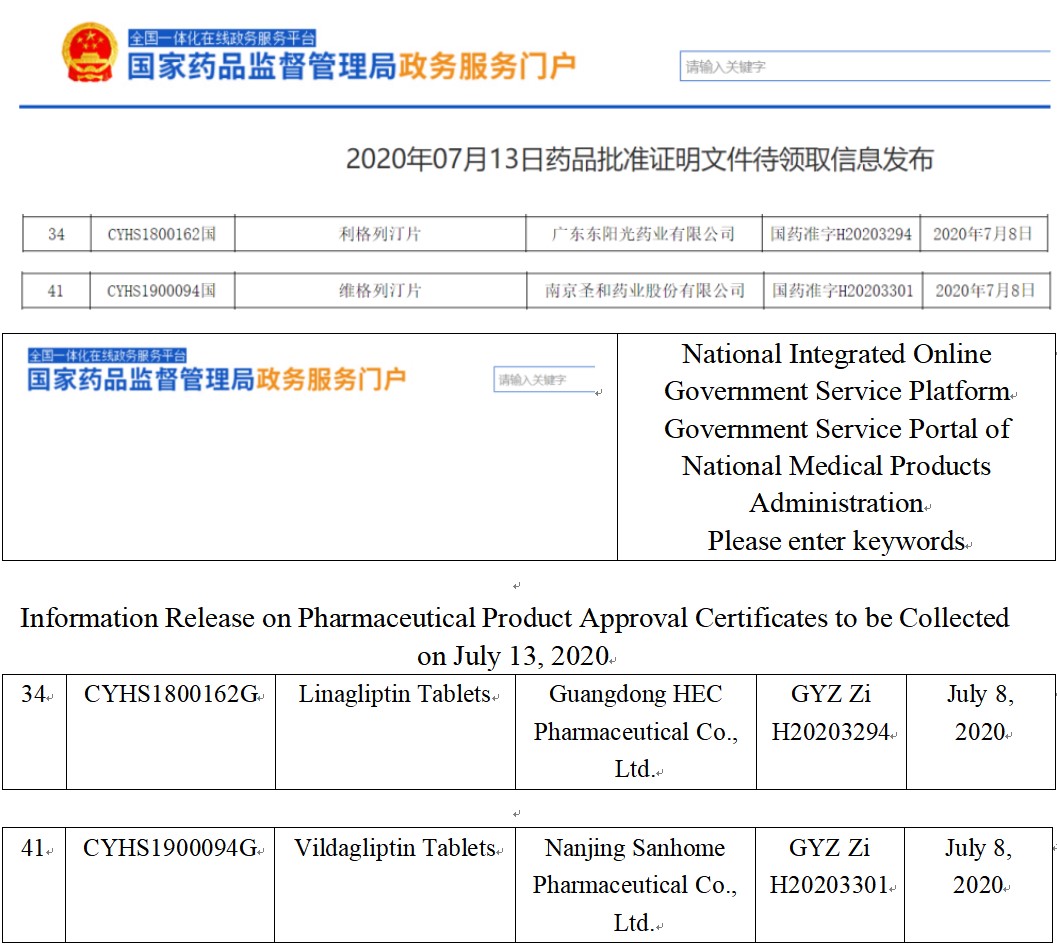
Fig. I Linagliptin and Vildagliptin Approval Information
(Screenshot of CDE website)
Let’s first check the approval of Guangdong HEC Pharmaceutical’s Class 4 generic drug: Linagliptin Tablets, the first generic of the variety in China. Co-developed by Boehringer Ingelheim (BI) and Eli Lilly, the original drug of linagliptin is a highly selective, potent DPP-4 inhibitor as an oral hypoglycemic used to treat type 2 diabetes as a monotherapy or in combination with metformin or metformin and sulfonylureas. The original drug was approved for marketing in China in Apr. 2013 (trade name: Trajenta), and the related market has been occupied by Boehringer Ingelheim (China) Investment Co., Ltd. The drug approved this time is the first generic of linagliptin in China, therefore, it has received great attention.
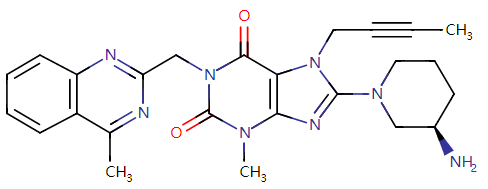
Fig. II Linagliptin Structural Formula
According to the Center for Drug Evaluation, NMPA (CDE website), BI has filed two supplementary applications for Linagliptin Tablets this year. Furthermore, a Class 4 generic drug application of CSPC Ouyi Pharmaceutical Co., Ltd. has also been filed.
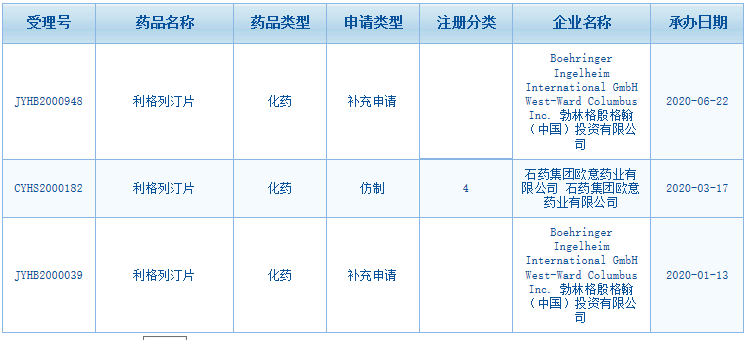
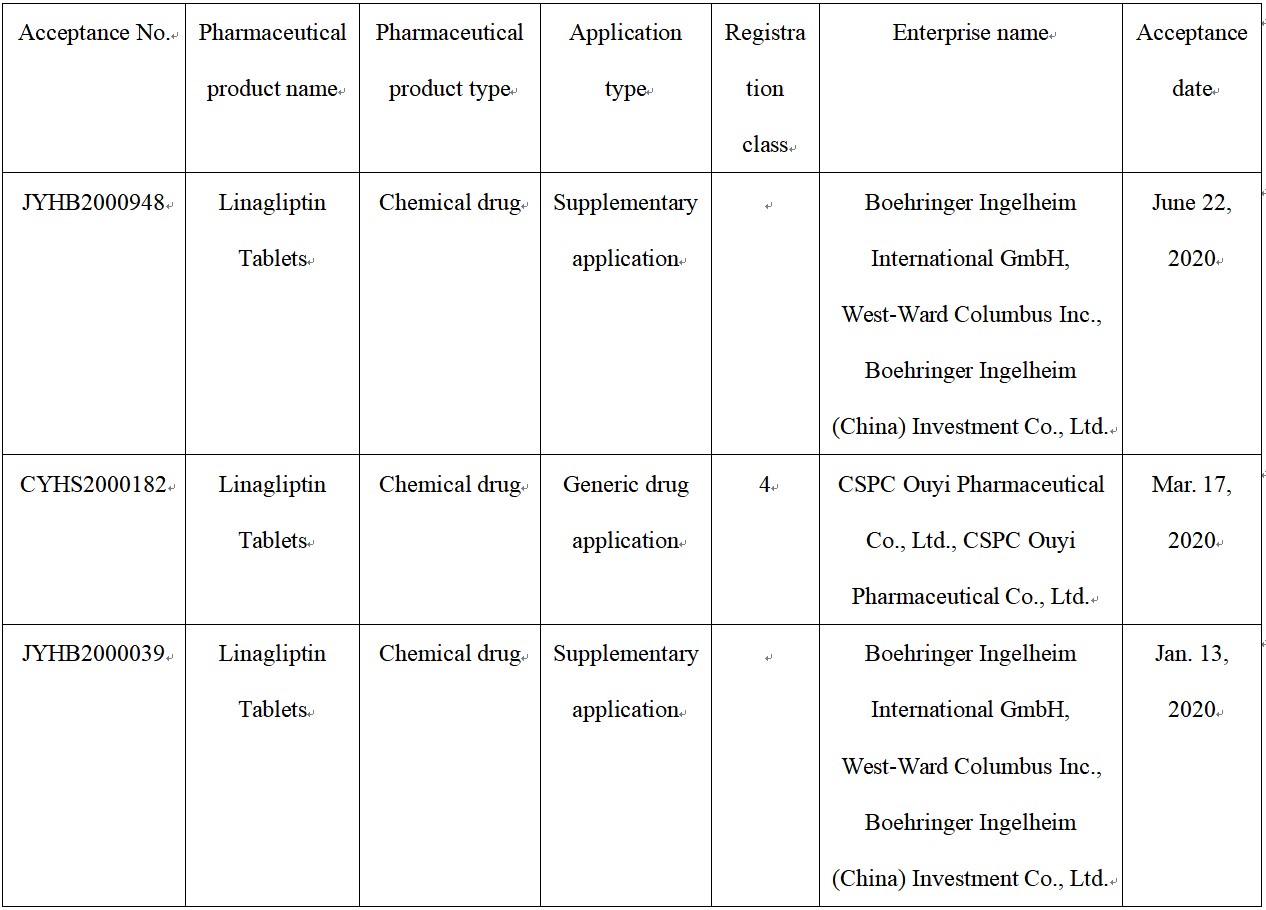
Fig. III Linagliptin Tablets Acceptance Information on CDE Website
Next, let’s check Nanjing Sanhome Pharmaceutical’s Class 4 generic drug: Vildagliptin Tablets. Originally developed by Novartis, vildagliptin is also a DPP-4 inhibitor as an oral hypoglycemic used to reduce the blood sugar of patients with type 2 diabetes, with advantages including not easily inducing hypoglycemia and producing no effect on patients’ weight. Sales of vildagliptin reached nearly USD1.3 billion in 2019. The drug first entered the Chinese market in 2011 and is used to treat patients with type 2 diabetes in combination with metformin when metformin, as a monotherapy, has poor blood sugar control.
Besides the developer Novartis, vildagliptin generic drugs of Hansoh, Qilu and Beijing Tide have been approved for marketing, and Nanjing Sanhome Pharmaceutical is the fourth medical supplies manufacturer approved to market it. Furthermore, according to the CDE website, vildagliptin generic drug jointly applied for by Tianjin Hanrui Pharmaceutical Co., Ltd. and Shandong Fenghuang Pharmaceutical Co., Ltd. has also been accepted, therefore, the market competition for the drug will be fierce in the future.
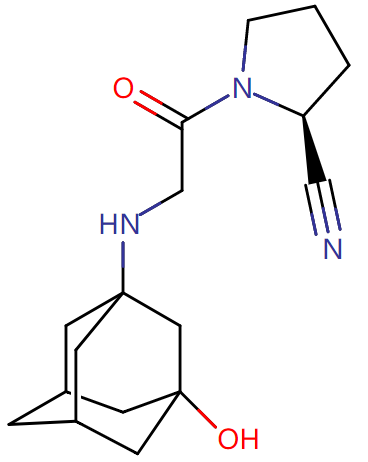
Fig. Vildagliptin Structural Formula
There are so far 5 DPP-4 inhibitors marketed in China, including sitagliptin, saxagliptin, vildagliptin, alogliptin benzoate, and linagliptin. With the first generic of linagliptin approved , those 5 DPP-4 inhibitor hypoglycemics will all have original drugs and generic drugs marketed in China.
Therefore, the DPP-4 inhibitor market competition will be fierce and increasingly intensified. However, according to an insider, those 5 DPP-4 inhibitor hypoglycemics have entered the NRDL (National Reimbursement Drug List of China) in 2017, with overall sales growing year by year, greatly benefiting the enterprises possessing those drugs, therefore, the marketing of new generic drugs might not reduce the sales of related enterprises, on the one hand; the marketing of generic drugs will generally lead to price reductions to benefit patients in terms of treatment costs, on the other hand.
References:
1. CDE website;
1. Drugbank;
2. http://www.nmpa.gov.cn/WS04/CL2455/378682.html.
Miling, Master of Pharmacy, majored in biopharmaceutical, has long been engaged in new drug research and development, focusing on the analysis of trends in the domestic and overseas drug market, and is good at the research and development of biological drugs and small molecule drugs.
-----------------------------------------------------------------------
Editor's Note:
To become a freelance writer of PharmaSources.com,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@imsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


