July 14,2020
Invivoscribe, Inc., an industry pioneer and global leader in the field of
precision diagnostics announces the launch of a fully integrated drug development engine. This new engine will combine with its in-house expertise in
diagnostics development and worldwide access to patients and clinical testing
capabilities to accelerate drug development.
Invivoscribe's therapeutics division also announced today that the company
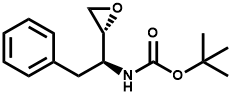
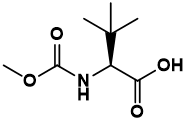
has in-licensed technology involving a preclinical molecule and associated small
molecules from Domainex Ltd. Domainex is a rapidly growing, well established
integrated drug discovery service company in the UK that has utilized its novel,
proprietary technologies to develop a first-in-class small molecule program
against a difficult to target enzyme. Under the license agreement, Invivoscribe
will develop and commercialize certain molecules generated by Domainex as oral
immunotherapy agents to treat hematological malignancies, including acute
myeloid leukemia. The overall financial aspects of the deal include an upfront
payment and subsequent milestone payments based on various phases of drug
development, regulatory submission and commercialization, including royalties on
the resulting commercial products.
"The launch of Invivoscribe Therapeutics was an obvious next step for
Invivoscribe. We have assembled an extraordinary team of drug development
experts with decades of experience leading discovery, preclinical development,
and directing pharmaceutical sciences. They are well versed in taking small
molecules through all phases of candidate selection into human trials. They've
joined our diagnostics, bioinformatics, and regulatory teams that have years of
experience using our tests to assist pharma companies in getting their drugs
approved worldwide, and our international network of LabPMM clinical
laboratories with ready access to oncology patients. All of our interactions
with Domainex have been extremely positive and we are impressed with the
molecules they have discovered," said Jeffrey
Miller, CSO & CEO of Invivoscribe.
Dr. Tom Mander, CEO of Domainex, added, "This
license agreement reflects the extensive capabilities of Domainex to generate
molecules with therapeutic potential. We are delighted that Invivoscribe has
decided to invest in moving the program through drug development ultimately to
approval and commercialization. This deal is in keeping with our strategy to
grow rapidly our drug discovery service business to enrich the pipelines of our
partners. I want to thank all of our scientists who have worked on the program
and acknowledge the support we have received along the way. I wish Jeff and his
team every success with the program and the launch of their new division."





 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story



















 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








