PharmaSources/XiaoyaowanJune 24, 2020
Tag: Influenza , baloxavir marboxil , oseltamivir
According to the website of the CDE on June 10, the marketing applications of Roche for its new drug Baloxavir Marboxil Tablets in China have been accepted by the CDE. This blockbuster new anti-influenza drug will soon bring a more convenient anti-influenza treatment experience to people with influenza in China.
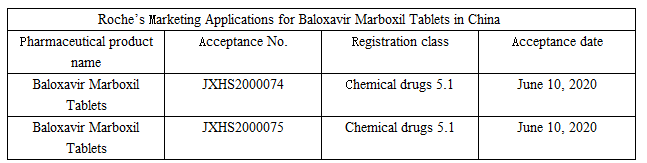
Source: CDE
Anti-influenza therapeutic regimen with a brand-new mechanism of action
As RNA viruses, influenza viruses are classified into types A, B, C and D based on differences in proteins and matrix proteins. In the drug treatment field, specific anti-influenza drugs can be used for the prevention of influenza and the relief of influenza symptoms in patients. In the current anti-influenza drug area in China, the neuraminidase inhibitor: oseltamivir phosphate is the anti-influenza drug of choice, accounting for a major market share.
Roche’s baloxavir marboxil is an ester prodrug, which is hydrolyzed into the active substance baloxavir marboxil in the body and achieves the anti-influenza virus purpose by inhibiting polymerase acidic (PA) endonuclease necessary for the transcription of the influenza virus genome. It has strong inhibitory activity against influenza A and B viruses and possesses a brand-new anti-influenza mechanism of action.
The only single-dose oral drug approved for influenza treatment at present
First developed by Shionogi & Co., Ltd., Baloxavir Marboxil Tablets received Japan PMDA’s priority review designation in Nov. 2015 for single-dose treatment of acute influenza in patients aged ≥12 years without complications and was later approved for marketing in Japan in Feb. 2018 through accelerated approval for the treatment of influenza A and B, with the trade name Xofluza.
Roche reached a cooperation agreement with Shionogi in Feb. 2016 to be responsible for the R&D of the drug outside Japan and Taiwan, China Region of China, and have the right to commercially promote it outside Japan and Taiwan, China Region of China.
Roche’s Genentech received the FDA’s priority review designation for Baloxavir Marboxil Tablets in June 2018 and the approval for marketing it in Oct. 2018, with the trade name Xofluza.
In terms of fighting influenza, Baloxavir Marboxil Tablets can reduce the relief time of influenza symptoms by more than one day with just a single dose taken orally, which brings a more convenient anti-influenza therapeutic regimen for patients. By comparison, the preferred anti-influenza in China: oseltamivir phosphate requires patients to take the drug twice a day for 5 days. Xofluza is the first and currently the only single-dose oral drug approved for the treatment of influenza and also the first anti-influenza virus drug with a brand-new mechanism of action approved by the FDA in the recent 20 years.
Clinical trial layout of Roche in China
Roche has begun the marketing plan of Baloxavir Marboxil Tablets in the Chinese market since the end of 2018. To date, two clinical trials of Baloxavir Marboxil Tablets have been completed in China for indications that include influenza as well as influenza A or B virus infection.
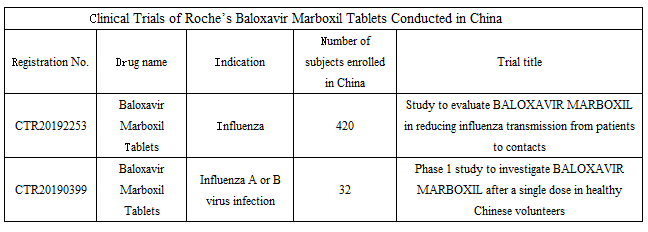
Wherein, the Phase IIIB, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Clinical Efficacy Study of Baloxavir Marboxil for the Reduction of Direct Transmission of Influenza From Otherwise Healthy Patients to Household Contacts plans to enroll 3,160 participants in the world, with 420 Chinese participants. Roche’s marketing applications for Baloxavir Marboxil Tablets in China this time are presumably based on the results of the said Phase IIIB clinical study.
Wherein, the Phase IIIB, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Clinical Efficacy Study of Baloxavir Marboxil for the Reduction of Direct Transmission of Influenza From Otherwise Healthy Patients to Household Contacts plans to enroll 3,160 participants in the world, with 420 Chinese participants. Roche’s marketing applications for Baloxavir Marboxil Tablets in China this time are presumably based on the results of the said Phase IIIB clinical study.
Furthermore, the clinical trial application for Baloxavir Marboxil Granules for Oral Suspension has also been approved in China, with no clinical study initiated for the time being.
Anti-influenza drug market in China in the future
Roche’s oseltamivir was approved for entry into China in Nov. 2001 with the trade name Tamiflu. Oseltamivir of Yichang HEC Changjiang Pharmaceutical and Shanghai Zhongxi Sunve Pharmaceutical was successively approved for marketing in 2006, with the trade name being Kewei and Aoerfei separately.
According to the 2019 performance report of HEC, the sales revenue of Kewei (oseltamivir granules and capsules) reached RMB5.93 billion in the year, growing by 163.9% year on year, accounting for 95.3% of the overall market of the company. It is noteworthy that HEC’s Kewei has now captured a major market share of oseltamivir in China thanks to its successful market operation.
According to the 2019 performance report of HEC, the sales revenue of Kewei (oseltamivir granules and capsules) reached RMB5.93 billion in the year, growing by 163.9% year on year, accounting for 95.3% of the overall market of the company. It is noteworthy that HEC’s Kewei has now captured a major market share of oseltamivir in China thanks to its successful market operation.
With Roche advancing the marketing process of Baloxavir Marboxil Tablets in the Chinese market, HEC’s Kewei will be faced with market competition pressure from this blockbuster new anti-influenza drug of Roche.
With Roche advancing the marketing process of Baloxavir Marboxil Tablets in the Chinese market, HEC’s Kewei will be faced with market competition pressure from this blockbuster new anti-influenza drug of Roche.
Xiaoyaowan, a pharmaceutical industry practitioner, a word carrier in the We-media era focusing on changes of the pharma industry.
-----------------------------------------------------------------------
Editor's Note:
To become a freelance writer of PharmaSources.com,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@imsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


