PharmaSources/ZongqinJune 17, 2020
Tag: Targeted Oncology Drugs , NTRK , cancer drug
Introduction
According to the official website of the CDE recently, InnoCare Pharma’s ICP-723 tablets has received a clinical trial implied permission, which is planned to be used to treat advanced or metastatic solid tumors with NTRK gene fusions. As a second-generation TRK small-molecule inhibitor, ICP-723 will be used to treat NTRK fusion-positive cancer patients with different tumor types and also patients with acquired resistance due to the first-generation TRK inhibitor treatment. InnoCare Pharma, upon receipt of the IND approval, will start clinical trials in China for multiple cancer types with NTRK gene mutations.
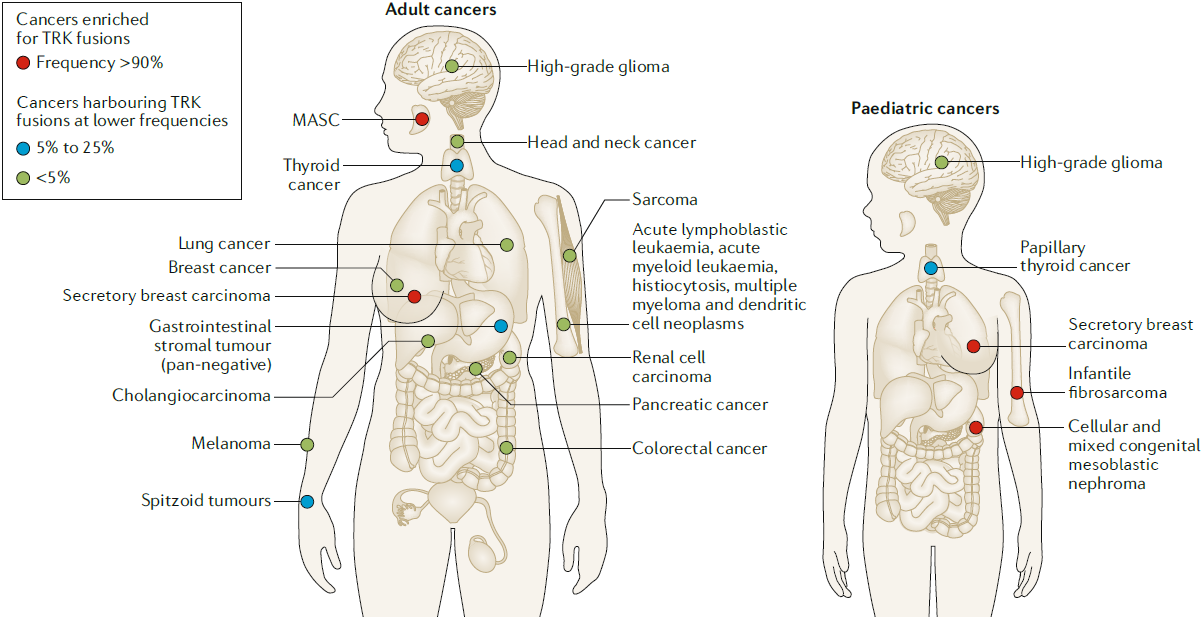
Fig. I Distribution and Frequency of NTRK Fusions in Adult and Paediatric Tumors [1]
Marketed drugs that target NTRK
A total of 4 drugs that can act on NTRK target have been approved for marketing; wherein entrectinib and larotrectinib belong to the first-generation TRK inhibitors, with good selectivity and strong specificity, mainly used to treat solid tumors with NTRK gene fusions, being another “broad-spectrum” anti-cancer drug irrelevant to tumor types.
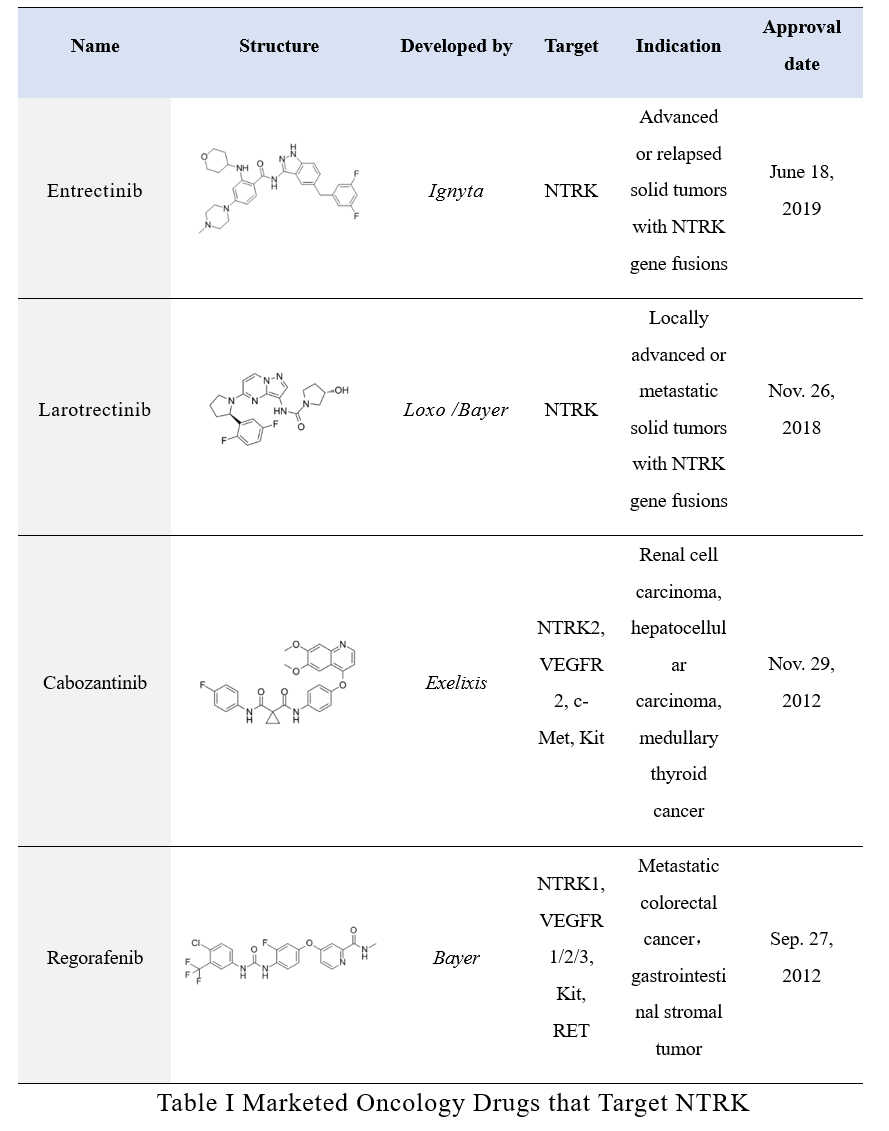
Entrectinib
The broad-spectrum new anti-cancer drug entrectinib is a new, orally effective, tyrosine receptor kinase (TRK) inhibitor with central nervous system (CNS) activity, which is used as targeted therapy of solid tumor patients with NTRK-encoded fusion protein or proto-oncogene tyrosine-protein kinase 1 (ROS 1) gene-encoded fusion protein and anaplastic lymphoma kinase (ALK)-encoded protein gene rearrangement.
The European Medicines Agency (EMA) granted the Priority Medicine (PRIME) designation to the product on Oct. 22, 2017 according to the results of 3 phase 1 and 1 phase 2 clinical trials conducted by Ignyta, Inc. Roche (which has acquired Ignyta) entered into a license agreement with Chugai Pharmaceutical Co., Ltd. (Chugai) on July 15, 2018 to authorize the latter with full rights to develop and apply for the marketing of the product in Japan. On Mar. 15, 2019, Chugai filed the marketing application for entrectinib capsules to the Pharmaceuticals and Medical Devices Agency (PMDA) of Japan. And entrectinib was first approved for marketing in Japan on June 18, 2019. Roche’s subsidiary Genentech filed a new drug application (NDA) to the U.S. FDA for entrectinib capsules in May 2019, and FDA approved the marketing on Aug. 15, 2019 through accelerated approval.
Entrectinib is the third broad-spectrum oncology drug following the marketing of pembrolizumab injection approved by the U.S. FDA on Sep. 4, 2014 and of larotrectinib thin membrane coated tablets. It is indicated for nearly 20 tumors including CNS tumor, neuroendocrine tumor, salivary gland tumor, pancreatic cancer, non-small cell lung cancer (NSCLC), thyroid cancer, colorectal cancer, bile duct cancer, and breast cancer, etc.
Larotrectinib
Co-developed by Bayer and Loxo Oncology, larotrectinib is the world’s first oral TRK inhibitor that was separately approved in the U.S. and EU in Nov. 2018 and Sep. 2019 and is used to treat locally advanced or metastatic solid tumor adult and child patients with NTRK gene fusions.
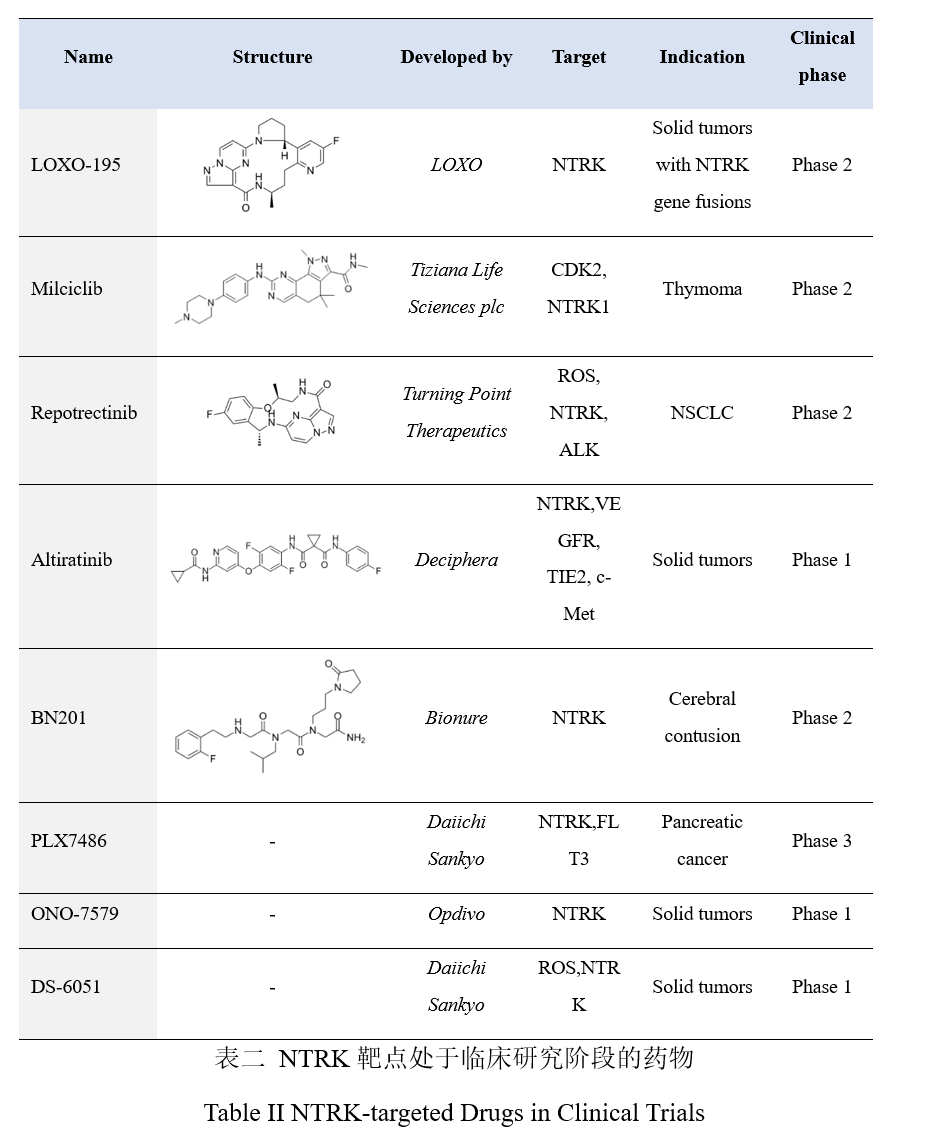
Regarding the resistance to larotrectinib, according to previous reports, the possible mechanisms leading to primary resistance include NTRK3 G623R mutation, NTRK molecule fusion without pathogenesis-related protein expression, and false positive in the detection; mechanisms leading to the secondary resistance include NTRK kinase domain mutation or bypass channel activation. According to the existing clinical data, specific targeting resistance mechanisms may be overcome by the next generation of NTRK inhibitors, such as selitrectinib and repotrectinib.
NTRK-targeted drugs in clinical trials
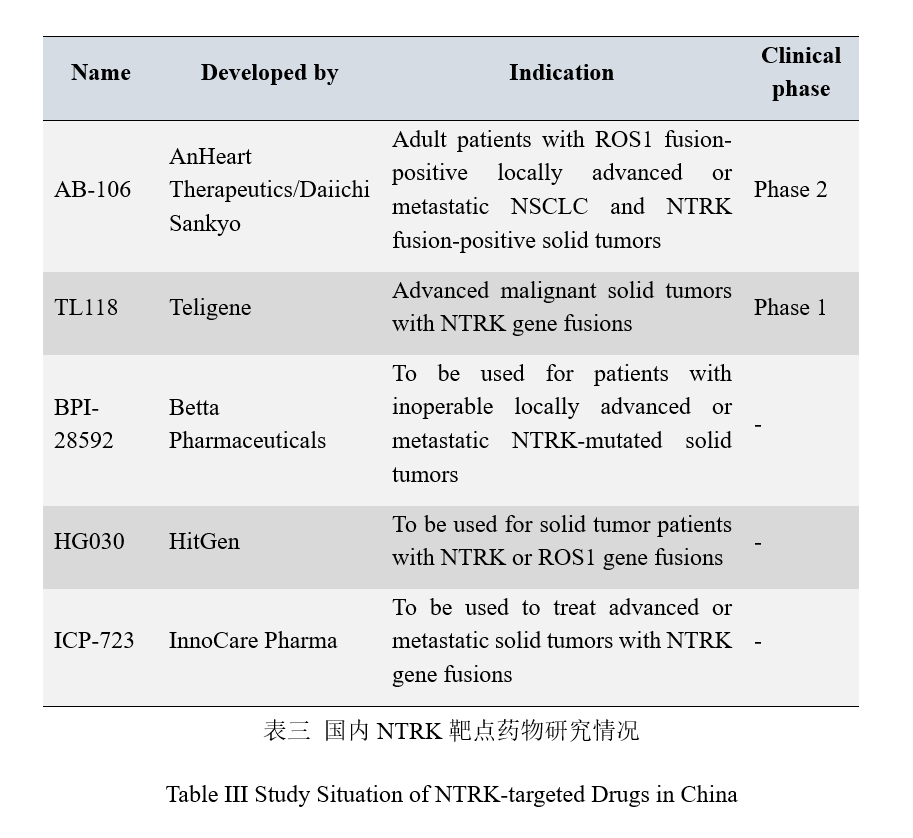
NTRK-targeted drugs in China
As an innovative oncology drug in development introduced by AnHeart Therapeutics from Daiichi Sankyo, AB-106 is currently in phase 2 clinical trial and leading in the NTRK target study progress in China. According to the preclinical study, AB-106 can inhibit ROS1 tyrosine kinase and NTRK tyrosine kinase activity in a low concentration and also has inhibitory activity against the most common ROS1-G2032R mutant resistant to crizotinib and mutant partially resistant to NTRK inhibitor. Many medical supplies manufacturers are making researches on it.
Teligene registered to start phase 1 clinical trial of TL118 capsules in advanced malignant solid tumor patients with NTRK gene fusions in Aug. 2019.
Conclusion
In the era of precision treatment, more and more rarely seen mutations have been discovered such as NTRK fusions with the updating of the second-generation sequencing technique and the optimization in clinical practice; homotherapy for heteropathy has been well applied to enable more patients to obtain appropriate personalized treatment. The emergence of broad-spectrum oncology drugs distinguished based on biomarkers instead of tumor locations, such as entrectinib and larotrectinib, is a classic case of “precision therapy” guiding anti-cancer drug R&D. In China, the studies of NTRK-targeted therapeutic drugs are still at the preparatory stage and their future is worth looking forward to.
References
1. Nature Reviews Clinical Oncology 2018, 15, 731-747.
2. Drugs 2019, 79(13), 1477-1483.
3. The Lancet Oncology 2020, 21, 531-540.
4. Cancer Discovery 2017, 7(9), 963-972.
5. Cancer Discovery 2018, 8(10), 1227-1236.
-----------------------------------------------------------------------
Editor's Note:
To become a freelance writer of PharmaSources.com,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@imsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


