PharmaSources/LaoxueJune 05, 2020
Tag: Bottle , COVID-19 , Vaccine
The National Medical Products Administration of China issued an announcement on the chemical drug injection consistency evaluation on May 14.
The first human-use P3 biomedical production workshop project has been successfully delivered the other day, marking the birth of the world’s largest COVID-19 vaccine workshop, which is expected to produce 100 million doses annually and possess the production level to meet large-scale urgent use and future routine vaccination.
According to tje media report earlier, there were only 200 million vaccine bottles left in the world, which might be not enough, and the bottle supply would not be guaranteed even if the vaccine was invented.
Taken conjointly, the three messages can be used to predict a bullish global stock market for pharmaceutical glass because packaging materials are also a key in the consistency evaluation and required to be not inferior to those of the original drugs.
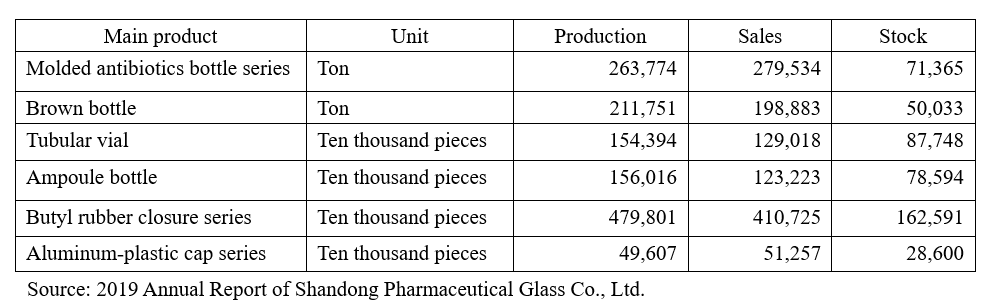
Let’s first look at the number of pharmaceutical glass bottles of major Chinese enterprises in stock: the tubular vial stock and ampoule bottle stock of Shandong Pharmaceutical Glass were separately 877.48 million pieces and 785.84 million pieces.
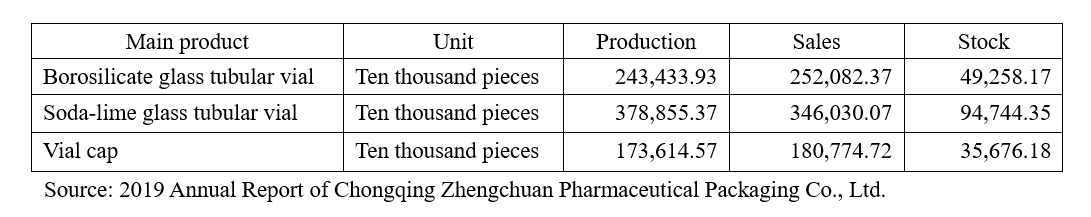
The borosilicate glass tubular vial stock and soda-lime glass tubular vial stock of Zhengchuan were separately 492.58 million pieces and 947.44 million pieces, and it’s specially mentioned in Zhengchuan’s report that the company intentionally stocked up in 2019 based on the market judgement.
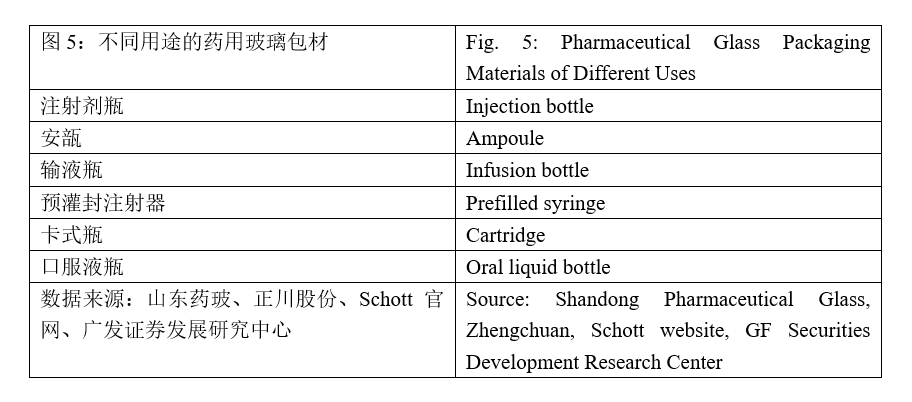
Assuming that the COVID-19 vaccine will be successfully developed, the storage containers will necessarily be required no matter in which country it will be first marketed. The existing containers of vaccines include ampoule bottles, cartridges, and prefilled syringes, etc.
As a cheap bottle type, ampoule bottles are currently the main storage method for veterinary drugs and some human drugs, however, they have a very evident defect that cannot be avoided, which is that the bottle body needs to be broken when to use the drug, possibly causing shards of glass to enter the drug or even be sucked by the syringe to eventually enter the human body. As a result, expensive pharmaceutical products rarely use ampoule bottles at present. Then pharmaceutical packaging equipment is quite important for these pharmaceutical products.
Ampoule bottles also need to be sealed after filling, however, tubular vials or those with rubber closures need to be crimped, therefore, they cannot be produced with the same filling equipment. The future trend of large volume parenterals is bagging, while the trend of vaccines is prefilling.

According to the search on the internet, the COVID-19 vaccine of Sinopharm also uses the prefilling technology, which has an apparent benefit: no separate syringe required. Conventional vaccine injection will produce three medical wastes: syringe, vaccine bottle, and absorbent cotton, while prefilling technology directly combines syringe and vaccine.
The stock price of Zhengchuan has significantly risen in recent one month as boosted by good news.
Shandong Pharmaceutical Glass’ stock declined on May 15, however, its stock price has also risen in recent one year.
Due to the shortage of COVID-19 vaccine, the vaccine may not be stored in a single form but is likely to be stored in penicillin bottles and ampoule bottles, etc., which is a test to glass products of different materials. The common vaccine bottles are neutral borosilicate glass with good resistance to thermal shock and resistance to water, which is often used to package various injections and blood and vaccine products, etc. Low borosilicate glass can reach Level 1 water resistance under 121℃, however, the water resistance of its internal surface cannot be guaranteed to reach intended effects, and it can be used to store pharmaceutical products with low requirements for chemical stability, such as oral liquid, and common antibiotic powder for injection, etc.; its development prospects are restricted due to its acid-base intolerance. However, vaccines are pharmaceutical products directly injected into the human body without dilution and have requirements for pH, and low borosilicate glass may also be used.
Internationally, neutral borosilicate glass tube enterprises include Nipro, NEG, and SCHOTT, etc. which have a large number of registration certificates and records filed. In China, such enterprises mainly include Shandong Pharmaceutical Glass and Zhengchuan, with high technical barriers. The investment in pharmaceutical glass R&D in China may be simulated by this opportunity. The vaccine packaging materials will be unlikely to be out of stock after the vaccine is marketed because multiple packaging forms can relieve the pressure. Following vaccine marketing is the development of cold chain transportation industry and medical waste treatment, etc., which may be the future pharmaceutical investment directions.
References:
Pharmaceutical Glass Industry Interpretation, GF Securities
2019 Annual Report of Chongqing Zhengchuan Pharmaceutical Packaging Co., Ltd.
2019 Annual Report of Shandong Pharmaceutical Glass Co., Ltd.
-----------------------------------------------------------------------
Editor's Note:
To become a freelance writer of PharmaSources.com,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@imsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


