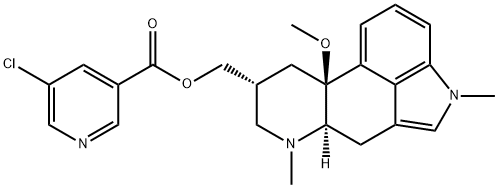
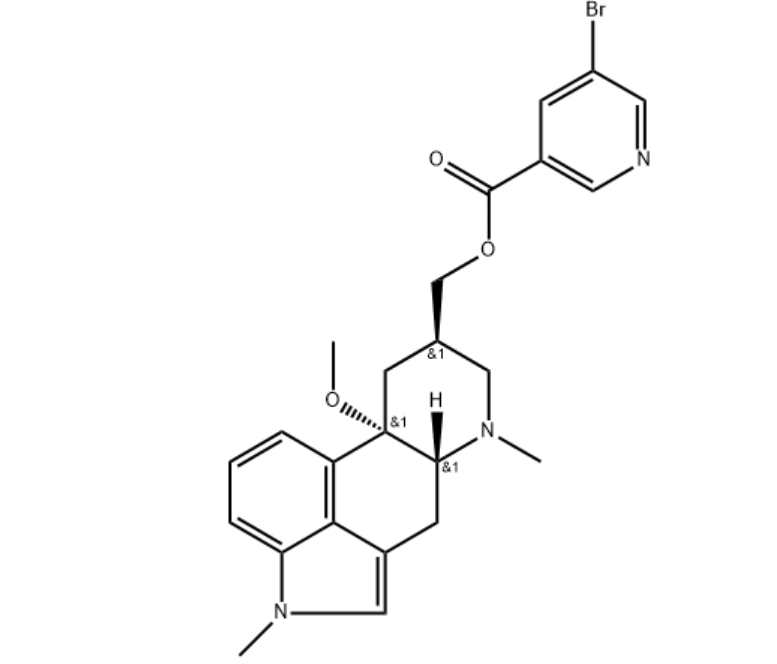
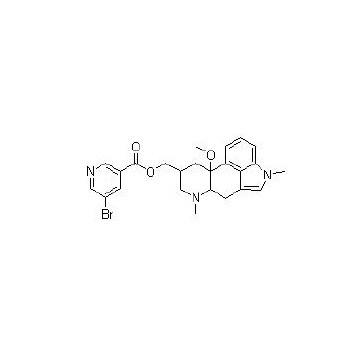
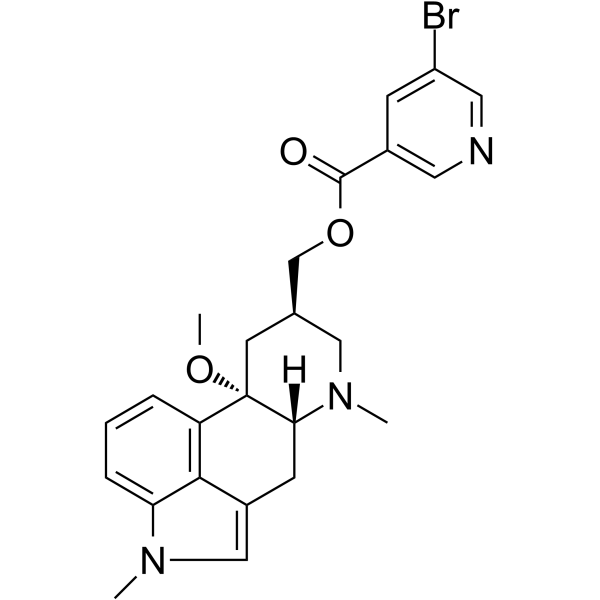
The National Institute for Health and Care Excellence (NICE) has now published final guidelines endorsing the NHS use of Teva’s Ajovy (fremanezumab) to prevent migraine.
The decision means use of the drug will be funded by the NHS in adults with chronic migraine who have not responded to at least three prior preventive treatments.
In this subset of patients, clinical trial evidence shows that Ajovy works better than best supportive care in both episodic and chronic migraine. However, it is unclear if fremanezumab works better than botulinum toxin type A, NICE said.
The anti-CGRP preventive therapy is the first of-its-kind to be recommended by the Institute, offering monthly or quarterly dosing options, with the choice to self-inject once patients are trained.
Its approval in April last year was based on two pivotal Phase III clinical trials, in which many patients on the treatment experienced significant reductions of at least 50% in the number of monthly migraine days with reduction observed as early as week one.
The list price of Ajovy is £450.00 per 225mg injection (£1,350 per 675mg) excluding VAT, but the company has agreed a commercial arrangement under which the drug is provided to the NHS at a confidential discount.
One in seven UK adults is affected by migraine (over 7.2 million people) and women are three times more likely to be affected than men.





 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story





























 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








