Lin ZhangJune 01, 2020
Tag: Repurposed Drug , New Indication , FDA
Repurposing drugs is not new to the pharmaceutical industry. There are numerous effective therapeutic agents whose side effect profiles have been documented over long-term use in clinical settings that are potential candidates for repurposing. Moreover, substantial costs and slow pace of new drug discovery and development, repurposing of 'old' drugs to treat both common and rare diseases is increasingly becoming an attractive proposition because it involves the use of de-risked compounds, with potentially lower overall development costs and shorter development timelines. In fact, approximately two-thirds of new drug applications submitted to the FDA are for modifications of existing drugs or their indications.(1)Classic life-cycle management of a branded drug often includes extending the value and life of the drug by innovative strategies such as reformulation, finding new indications, or rediscovering the inherent value of an old drug, all of which are intended to provide added benefit to patients.
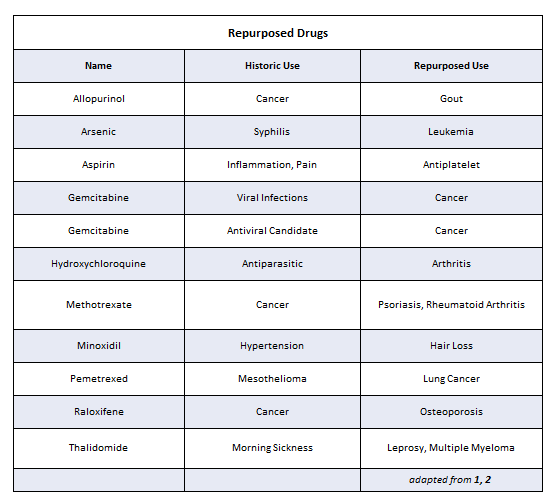
Repurposed Drugs
New applications or indications for drugs can be discovered systematically using sophisticated computer programs or serendipitously by noting unexpected, but beneficial, side effects of treatment. In some cases, targets of a known drug that are clinically relevant in one biologic pathway or disease may be relevant in other pathways or diseases as well. It is also possible to take advantage of drugs that interact with multiple targets by developing a new indication where a secondary target is more relevant. A partial list of well-known repurposed drugs is shown in Table 1.
Older drugs that have been overshadowed by advances in new drug development technologies, such as rational structure-based drug design and high-throughput screening (drug discovery experimentation that uses automated systems), may be revitalized when newer drugs either do not live up to their therapeutic potential or elicit an increased risk-to-benefit ratio over time.
Many medical and surgical supplies may benefit from new and innovative technologies that improve drug delivery systems, such as chewable tablets, oral liquids, or conversion of intravenous to non-injectable forms of delivery. For example, a different route of administration, such as conversion from oral to transdermal, may lower the dose required, permit a new indication, or reduce toxicity. Conversion to extended-release technology decreases the dosing frequency, which may increase adherence and decrease side effects by altering intestinal absorption.
Recent Repurposed Drugs
2020 alone has already seen an extensive amount of FDA approvals for repurposed drugs. The COVID-19 pandemic saw the initial repurposing and then warned against use hydroxychloroquine as a potential indication for treatment of the virus due to the risk of heart rhythm problems as announced by the FDA late April,(1) having stated no significant improvements observed related to its use. A list of recent drugs that have been repurposed is given below:(3)
Imbruvica Capsules and Tablets
Braftovi (encorafenib) Capsules
Reblozyl (luspatercept-aamt) for Injection
Imfinzi (durvalumab) Injection
Triferic (ferric pyrophosphate citrate)
Taltz (ixekizumab) Injection
Eucrisa (crisaborole) Topical Ointment
Epclusa (sofosbuvir and velpatasvir) Tablets
Ofev (nintedanib) Capsules
Nerlynx (neratinib) Tablets
Procysbi (cysteamine bitartrate) Delayed-Release Capsules
Voltaren Gel (diclofenac sodium) Topical Gel
Ajovy (fremanezumab-vfrm) Injection
Dificid (fidaxomicin)
Ozempic (semaglutide) Injection
Keytruda (pembrolizumab) Injection
Repurposed Drugs for Targeted Therapies
One of the most successful multi-use drug today is adalimumab. Marketed under the names: Humira, Amjevita, adalimumab-atto, the drug was originally developed as a treatment for pain and swelling due to certain types of arthritis. However, it has found a variety of uses, being indicated for the treatment of: rheumatoid, psoriatic, juvenile idiopathic, ankylosing spondylitis, plaque psoriasis, Crohn’s disease, pediatric Crohn’s disease, ulcerative colitis, and hidradenitis suppurativa. (4)
Spironolactone (Aldactone), a potassium-sparing diuretic that acts as an aldosterone receptor antagonist, is an example of an old drug that has seen a recent resurgence of interest. With the introduction of angiotensin-converting enzyme (ACE) inhibitors—which decrease urinary excretion of potassium by blocking angiotensin II–mediated production of aldosterone—the use of spironolactone fell out of favor after a spike in patients with serious hyperkalemia was observed when ACE inhibitors and spironolactone were used concomitantly. However, spironolactone usage increased following publication of RALES (Randomized Aldactone Evaluation Study), which demonstrated a 30 percent lower risk of morbidity and mortality in patients with severe heart failure and systolic left ventricular dysfunction who had received low-dose spironolactone.
Minoxidil (Loniten) is an example of an old drug repurposed with a new indication and reformulation,which is a powerful peripheral vasodilator, is used in combination with other antihypertensives to treat severe or refractory high blood pressure. In early studies, sodium retention and hirsutism were noted as significant side effects of minoxidil therapy. In the late 1980s, a topical preparation of minoxidil (Rogaine) was cleared by the FDA for male pattern baldness and androgenic alopecia; in the mid-1990s, 5 percent and 2 percent topical solutions of minoxidil were approved as OTC products. Reformulation to a 5 percent topical foam-based vehicle was assessed in a randomized, blinded clinical study that demonstrated significant improvement in hair counts and subjective assessment of hair loss condition and good tolerability compared with placebo. Thus, the foam formulation was found to be comparable to the solution with respect to performance and had the added benefit of enhanced cosmetic acceptability.
The most recent repurposed drug is that the National Institutes of Health (NIH) begins today (May 14, 2020) clinical trial of hydroxychloroquine and azithromycin to treat infected patients with mild to moderate COVID-19 in the United States.(5) The study is to evaluate whether the malaria drug hydroxychloroquine, given together with the antibiotic azithromycin can prevent hospitalization and death due to COVID-19 as well as the safety and tolerability of the experimental treatment for people infected with SARS-CoV-2 since there are no specific and effective therapeutics approved by the FDA to treat people with COVID-19.
Conclusion
Drug repositioning represents a possible alternative to the new medical discovery which is an increasingly crucial component of the current drug R&D landscape. Drug repurposing can solve two issues: improved health-care impact and reduced health-care cost. Modern technologies and tools are increasingly available that could revolutionize the way scientists are able to identify existing drugs for repurposing and reformulation as a faster, cheaper, safer way to drive therapies to patients and as a method of creating a smarter way of new drug development without unacceptable side effects or safety events.
Many clinical trials are currently underway worldwide. It should be noted that 65 percent of the ongoing trials include repurposed drugs or existing medicines that are already on the market to treat other diseases for which there are currently no effective treatments.(6) Scientists can move forward very quickly as the drugs are already tested on human safety.
References
https://www.fda.gov
https://geneva-network.com,07/2017
www.Drugs.com.
www.Rxlist.com.
https://www.nih.gov/news-events/news-releases/nih-begins-clinical-trial-hydroxychloroquine-azithromycin-treat-covid-19
https://www.anticancerfund.org, 7 April 2020
About the Author:
Lin Zhang, M.D., senior director of a health care industry company in the United States. With the experience in clinical medicine, biotechnology, health industry and other fields, he is responsible for the research and development of plant medicine, functional food and health products. He was a clinician and worked for the National Cancer Institute, FDA and the National Cancer Center of Japan for many years.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


