PharmaSources/CaicaiMay 26, 2020
Tag: PDB , sample hospitals
According to the PDB data, the data of the top 50 drugs by sales to sample hospitals in China in 2019 have been fresh out recently, with the total sales of the top 50 reaching RMB76.414 billion and the minimum requirement for listing being RMB910 million.
Top 50 Drugs by Sales to Sample Hospitals in China in 2019
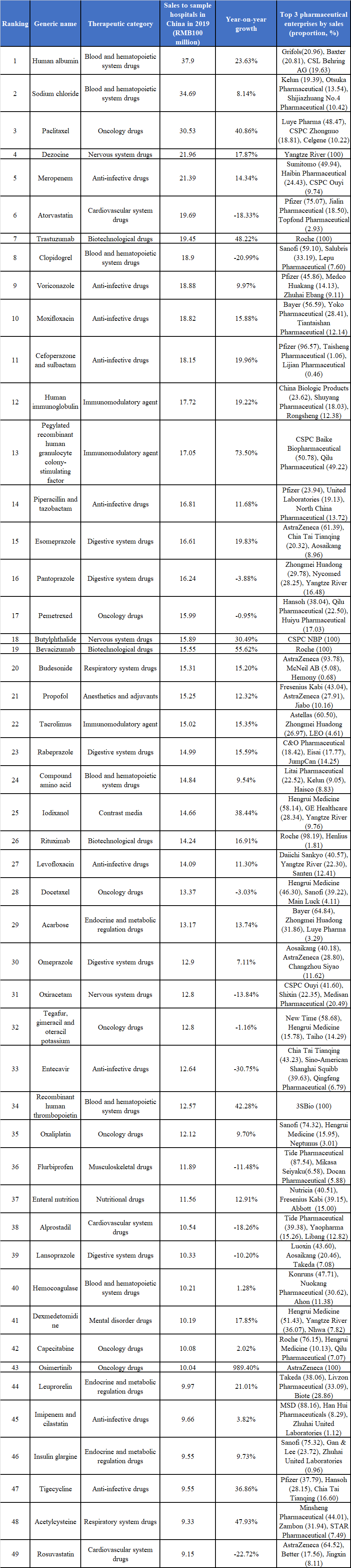
(Source: PDB; their actual sales in China may be several times their sales to sample hospitals)
From the perspective of growth: like a succession of waves
10 varieties among the top 50 grew by more than 30%, separately, osimertinib (989.40%), pegylated recombinant human granulocyte colony-stimulating factor (PEG-rhG-CSF) (73.50%), bevacizumab (55.62%), trastuzumab (48.39%), acetylcysteine (47.93%), recombinant human thrombopoietin (42.28%), paclitaxel (40.86%), iodixanol (38.44%), tigecycline (36.86%), and butylphthalide (30.49%).
Where, osimertinib had the fastest growth, with the sales to sample hospitals in China being RMB1.004 billion in 2019, growing by 989.40% year on year. The fast growth of those varieties was thanks to the large sales increase after entering the national reimbursement drug list (NRDL) of China, including osimertinib, PEG-rhG-CSF, bevacizumab, and trastuzumab, etc., on the one hand; it was also owing to the significant clinical advantages compared to the existing varieties, including paclitaxel (albumin-bound), iodixanol, and tigecycline, etc., on the other hand.
8 varieties among the top 50 had growth less than -10%, separately, entecavir (-30.75%), rosuvastatin (-22.72%), clopidogrel (-20.99%), atorvastatin (-18.33%), alprostadil (-18.26%), oxiracetam (-13.84%), flurbiprofen (-11.48%), and lansoprazole (-10.20%); wherein, entecavir, rosuvastatin, clopidogrel, atorvastatin, and flurbiprofen were varieties subject to procurement with target quantity, and the large decline of their prices resulted in the decline of their sales scale; alprostadil and oxiracetam were affected by the restricted adjuvant drug policy.

From the perspective of varieties: most were “old”
26 varieties among the global top 50 drugs by sales in 2019 were those marketed in 2010 and thereafter, accounting for more than a half, while, most of the top 50 drugs by sales to sample hospitals in China in 2019 were varieties marketed for years, and the varieties marketed in the recent decade were not many.
Wherein, the number of Chinese-produced innovative drugs listed is small, with only 3SBio's Class 1 biological product recombinant human thrombopoietin listed. The rest in the list were all imported original drugs or Chinese-produced generic drugs. Overall, the pharmaceutical product structure is slightly “aging”, there is great room for optimization, and the new superseding the old is expected to speed up.
Caicai, a Master of Pharmacy from Shanghai Jiaotong University, used to work in the Institute of Science and Technical Information. Currently as a practitioner in the drug surveillance system, she is good at interpreting industry regulations, pharmaceutical research developments, etc.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


