Lin ZhangMay 26, 2020
Tag: FDA Fast Track , COVID-19 , Vaccine
Introduction
The world has never faced a crisis like COVID-19 (SARS-CoV-2), a pandemic health emergency. As of May 7, 2020, there are over 3.6 million cases (70,581+ new) and over 251,446 deaths of COVID-19 worldwide. (1) In order to develop effective vaccine against COVID-19, the US Food and Drug Administration (FDA) has initiated new procedures to expedite the development of potentially safe and effective life-saving treatments, called the Coronavirus Treatment Acceleration Program (CTAP).
Department of Health and Human Services (DHHS) Secretary Azar indicated in an address that the FDA is announcing a new, comprehensive public-private approach to bring coronavirus treatments to the market as fast as possible. As part of this new program, the FDA is cutting red tape, redeploying staff, and working day and night to review requests from companies, scientists and doctors who are working toward therapies.
Under the CTAP, staff from the FDA’s Center for Drug Evaluation and Research and the Center for Biologics Evaluation and Research will provide regulatory advice for the development of novel vaccines against the COVID-19 pedantic, guidance and technical assistance as quickly as possible. As part of this work, the FDA is triaging requests from developers and scientists seeking to develop new drug and biologic therapies, getting the relevant FDA staff in touch with them and providing rapid, interactive input to get studies underway quickly. (2)

Advantages of the FDA Fast Track Process
According to the FDA, speeding the availability of drugs that treat serious diseases are in everyone's interest, especially when the drugs are the first available treatment or if the drug has advantages over existing treatments.
Fast-tracking allows manufacturers early and quicker access to valuable time-saving resources to advance a drug product to market as quickly and safely as possible. Because of the resources required to do this, the FDA does not grant Fast Track status lightly.
The designation is designed to aid in the development and expedite the review of drugs which show promise in treating a serious or life-threatening disease and address an unmet medical need. A Serious Condition label is based on whether the drug will affect such factors as survival, day-to-day functioning, or the likelihood that the disease, if left untreated, will progress from a less severe condition to a more serious one. For a drug to address an unmet medical need, the drug may be developed as a treatment or preventative measure for a disease which lacks an existing therapy. The type of information necessary to demonstrate an unmet medical need classification varies with the stage of drug development. In early development stages, nonclinical data, mechanistic rationale, or pharmacologic data will suffice. Later in the development stages, clinical data is strongly suggested. If there are already existing therapies, a fast track eligible drug must show some advantage over available treatment. These could include:
Showing superior effectiveness
Avoiding serious side effects of an available treatment
Improving the diagnosis of a serious disease where early diagnosis results in an improved outcome
Decreasing a clinically significant toxicity of an available treatment
Addressing an expected public health need.
If these requirements can be met sufficiently, the FDA will approve Fast Track status. Fast Track designation opens up a manufacturer and their drug product to some or all of the following:
More frequent meetings with the FDA to discuss the drug's development plan and ensure collection of appropriate data needed to support drug approval
More frequent written correspondence from FDA about such things as the design of the proposed clinical trials
Accelerated Approval or priority review if the requisite criteria are met. Accelerated approval is meant for drugs that demonstrate an effect on a surrogate, or intermediate endpoint reasonably likely to predict clinical benefit. Priority review shortens the FDA review process for a new drug from ten months to six months and is appropriate for drugs that demonstrate significant improvements in both safety and effectiveness of an existing therapy. A fast track application is automatically considered for both of these designations.
Rolling Review, which means that medical supplies manufacturers can submit completed sections of its New Drug Application (NDA) for review by FDA, rather than waiting until every section of the application is completed before the entire application can be reviewed. NDA review usually does not begin until the drug company has submitted the entire application to the FDA
Once a drug receives Fast Track designation, early and frequent communication between the FDA and a drug company is encouraged throughout the entire drug development and review process. The frequency of communication assures that questions and issues are resolved quickly, often leading to earlier drug approval and access by patients.
Novel Vaccines Which Could Be Fast-Tracked by FDA For The COVID-19 Pandemic
As of April 2020, the global COVID-19 vaccine R&D landscape is made up of 115 vaccine candidates, with 78 confirmed active and 37 unconfirmed. 73 of the confirmed active projects are currently at exploratory or preclinical stages. (3) The most advanced candidates have recently moved into clinical development, including mRNA-1273 from Moderna, Ad5-nCoV from CanSino Biologicals, INO-4800 from Inovio, and LV-SMENP-DC and pathogen-specific aAPC from Shenzhen Geno-Immune Medical Institute (4, Table 1). Among the confirmed active vaccine candidates, 72% are being developed by private/industry developers, with the remaining 28% of projects being led by academic, public sector and other non-profit organizations. Although a number of large multinational vaccine developers have engaged in COVID-19 vaccine development, many of the lead developers are small and/or inexperienced in large-scale vaccine manufacture. This increases the need for collaboration and access to resources.
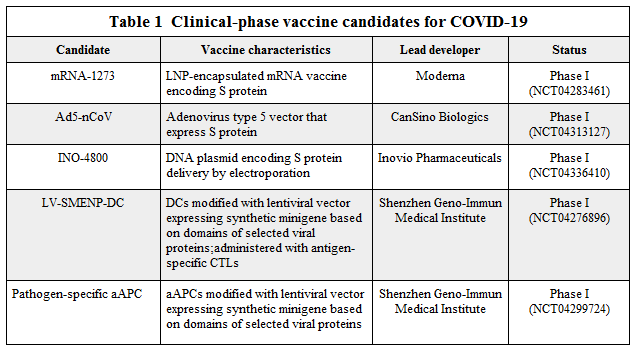
(Source: Thanh Le et al., 2020 Nature Reviews)
Conclusion
While the US FDA has initiated ground breaking changes in regulation to allow for the fast-tracking of promising vaccines to treat COVID-19, much work is still left to be done. With large pharma companies having the resources and ability to push forward into Phase I testing, which will look more favorable to FDA Fast Track status. Along the way, smaller corporations, universities and/or non-profits might struggle to see quick acceptance. To successfully combat this pandemic with the ability to target the right drug with the minimal potential side effects is a task ever present in the minds of the FDA regulators. Cooperation and an aggressive go-to-market strategy combined with FDA Fast Track benefits will lead to a quicker rollout of a vaccine(s) ready for mass production.
References
WHO:https://covid19.who.int
FDA: www.fda.gov Office of the (November 3, 2018). "Fast Track".
https://www.europeanpharmaceuticalreview.com/news/116125/fda-and-ema-offer-updates-on-covid-19-treatments-and-vaccines-in-development/. 2020, 1 April.
Nature Reviews: Drug Discovery. 2020, April 9th.
About the Author:
Lin Zhang, M.D., senior director of a health care industry company in the United States. With the experience in clinical medicine, biotechnology, health industry and other fields, he is responsible for the research and development of plant medicine, functional food and health products. He was a clinician and worked for the National Cancer Institute, FDA and the National Cancer Center of Japan for many years.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


