PharmaMar has announced the start of the APLICOV-PC clinical trial with Aplidin® (plitidepsin), for the treatment of patients with COVID-19, which has been authorized by the Spanish Medicines and Healthcare Products Agency (AEMPS).
This is a multicenter, randomized, parallel, open-label study to evaluate the safety profile and efficacy of three doses of plitidepsin in patients with COVID-19 requiring hospital admission.
Three hospitals from Madrid (Spain) will participate in the study. Three cohorts of patients with three different dose levels will be included in the study to assess the efficacy and safety of plitidepsin at each dose level administered in patients admitted to hospitals with COVID-19.
During this first stage, 27 patients will be recruited, to whom three doses will be administered. The viral load of the patients will be measured before and after the treatment, as well as a series of other parameters for clinical evolution. If the results were positive at this early stage, the trial would continue at the optimal dose after discussion with the regulator, with a larger cohort of patients.
On March 13th, the Company announced the results of in vitro studies of plitidepsin on the human HCoV-229E coronavirus, which has a very similar multiplication and propagation mechanism to SARS-CoV-2, as they both use the eEF1A protein for their reproduction. The studies were carried out at the National Biotechnology Center of the Spanish National Research Council (CSIC) (see press release).
Plitidepsin acts by blocking the protein eEF1A, present in human cells, which is used by SARS-CoV-2 to reproduce and infect other cells. By means of this inhibition, the expectation is that reproduction of the virus inside the cell is prevented, making this propagation to the rest of the cells unviable.
CPhI China 2020 To Be Postponed to Dec. 16-18th,
Register as Visitor to the 20th Edition of CPhI China!

-----------------------------------------------------------------------
Editor's Note:
En-CPhI.CN is a vertical B2B online trade platform serving the pharmaceutical industry,
for any copyright disputes involved in the articles,
please email: Julia.Zhang@imsinoexpo.com to motify or remove the content.





 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story

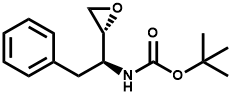
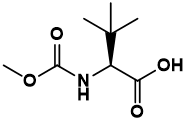




















 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








