PharmaSources/CaicaiApril 26, 2020
Tag: Novartis , indication , Secukinumab, , Editor's Picks
The marketing application (acceptance No. JXSS1900025) of a new indication of Novartis secukinumab in China has recently changed to Under approval and is expected to be approved soon. First approved by the NMPA for marketing in Mar. 2019, this will be the 2nd indication of secukinumab approved to treat ankylosing spondylitis.
Novartis Best-selling Drug in 2019
Secukinumab (trade name: Cosentyx) is a fully human IgG1 anti-IL-17A monoclonal antibody developed by Novartis. Approved by the FDA in 2015, it has now been marketed in more than 80 countries and regions including the EU countries and the U.S. and approved to treat plaque psoriasis, ankylosing spondylitis, and psoriatic arthritis, etc.
IL-17A is the core pathogenic factor that gets involved in the inflammation production and disease progression of psoriasis (PsO), psoriasis arthritis (PsA), and ankylosing spondylitis (AS). The production of IL-17A can be IL-23-dependent and non-IL-23-dependent and by various cells of the innate immune system and adaptive immune system. Secukinumab has shown lasting effectiveness and safety in various areas of PsO (including nail, scalp, palmoplantar area, and joint) by directly acting on different sources of IL-17A.
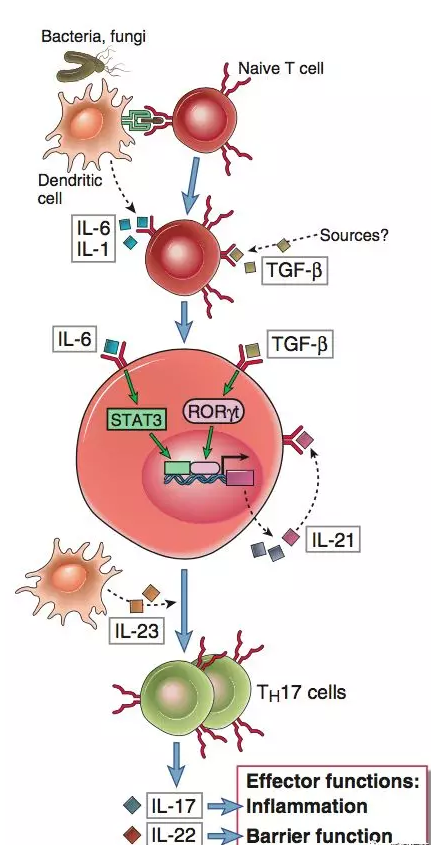
Secukinumab has become a rising star that has achieved repeated great results in PsO and AS. In particular, the success of the head-to-head clinical trial of secukinumab vs ustekinumab where secukinumab showed clinical benefits superior to ustekinumab in the PsO indication has gained secukinumab considerable fame. Secukinumab still maintained rapid growth in 2019 and became the best-selling drug of Novartis.
Secukinumab already became the best-selling drug of Novartis in 2019 only 5 years after its marketing, with global sales reaching USD3.55 billion in 2019, growing by 25% year on year.
Marketing of the first indication in China
As one of the first batch of overseas new drugs catering to clinical urgent needs in China, secukinumab was approved by the NMPA to treat moderate-to-severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy in Mar. 2019 and was formally marketed in China in May of the same year. If you are looking for online medical supply companies who manufacture this kind of drug in China, then Pharmasources would be your best choice.
According to the information on ClinicalTridals.gov, secukinumab has been completed two clinical studies that included Chinese patients, with the indication separately being plaque psoriasis and AS; wherein, the international multi-center phase 3 clinical trial for AS has been completed on Mar. 19, 2019.
The 2nd indication to be approved soon
The new indication for AS (acceptance No.: JXSS1900025) was applied for marketing in May 2019 and included in the priority review in August.
As a kind of chronic inflammation that occurs in the spine of patients, AS has a long course and will cause patients to lose most of their ability to act if it gets worse. Susceptibility gene and family history are the main pathogenic factors of AS. As a result, the number of Chinese AS patients is relatively stable.
According to a report of Frost & Sullivan, the number of Chinese AS patients increased from 3.76 million to 3.85 million from 2014 to 2018, with a compound annual growth rate (CAGR) of 0.6%; the number is expected to reach 3.955 million in 2023, with a CAGR of 0.5%; the number will continue to grow at a CAGR of 0.4% and reach 4.054 million in 2030.
Number of Chinese AS Patients from 2014 to 2030.
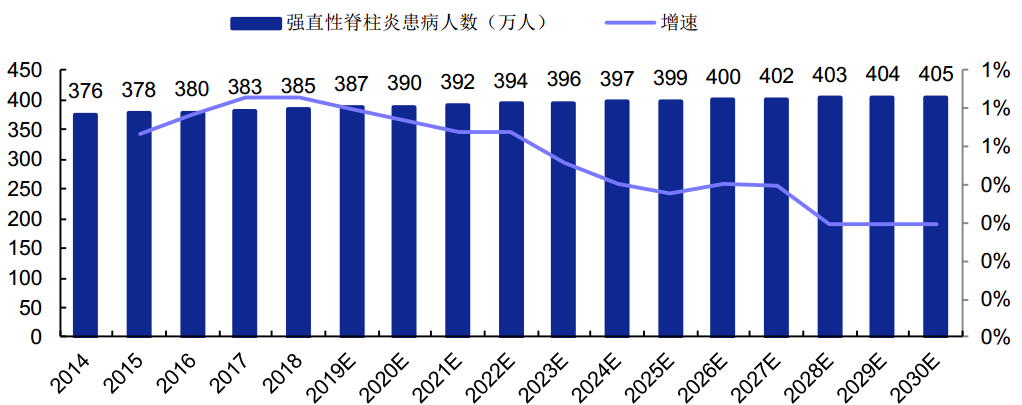
强制性脊柱炎患病人数(万人) | Number of AS Patients (10,000) |
增速 | Growth Rate |
(Source: Bio-Thera prospectus, Frost & Sullivan)
The safety and effectiveness of secukinumab are superior to those of most macromolecular drugs marketed in the area in China, however, the sales of secukinumab in China still face challenges. The Chinese-produced Yisaipu of Sunshine Guojian has the first-mover advantage and has been included in the national reimbursement drug list (NRDL) of China, with a market share of about 60.9% in the Chinese market (of rheumatoid + AS + psoriasis drugs) and annual sales of more than RMB1 billion, occupying the absolute dominance.
Caicai, a Master of Pharmacy from Shanghai Jiaotong University, used to work in the Institute of Science and Technical Information. Currently as a practitioner in the drug surveillance system, she is good at interpreting industry regulations, pharmaceutical research developments, etc.
-----------------------------------------------------------------------
Editor's Note:
To become a freelance writer of En-CPhI.CN,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@imsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


