PharmaSources/LaoxueApril 20, 2020
Tag: TCM , COVID-19 , Lianhua Qingwen
A pharmaceutical product supplementary application approval was spread on WeChat moments on the evening of Apr. 13, which was about Yiling Pharmaceutical’s revision of the “Functions” and “Usage and dosage” in the package insert of Lianhua Qingwen Capsules.
The approval conclusion of the National Medical Products Administration of China (NMPA) says: According to the related provisions of the Drug Administration Law of the People’s Republic of China and the COVID-19 treatment clinical practice, the prescription drug package insert is approved to be issued to Lianhua Qingwen Capsules. Besides the origina lly approved contents in the [Functions] of the prescription drug package insert, the following is added: "The drug can be used to treat fever, cough, and lassitude caused by mild and ordinary COVID-19 in the conventional treatment." Besides the originally approved contents in the [Usage and dosage], the following is added: "A course of 7-10 days for treating mild and ordinary COVID-19". Quality-standard-related contents in the [Functions] and [Usage and dosage] and the label contents shall be consistent with the corresponding contents in the package insert.
The approval of the NMPA also says: "The marketing authorization holder shall further accumulate clinical effectiveness and safety data, strengthen the collection of the pharmaceutical product’s adverse reaction information and take corresponding risk control measures. If unintended toxic reactions are discovered in the clinical use of the product, the marketing authorization holder shall conduct the toxicologic study depending on the circumstances to provide a reference for controlling the clinical use risks".
Lianhua Qingwen Capsules has received the clinical ethics approval for the "randomized, controlled clinical trial of treating COVID-19 with Lianhua Qingwen Capsules/Granules" early on Jan. 31, 2020, according to which, the control group would receive the conventional treatment while the trial group would receive conventional treatment plus Lianhua Qingwen Capsules/Granules, with the sample size both of 120 cases.
Responsible persons for the trial are all key persons during this epidemic: Zhong Nanshan, Zhang Boli, Li Lanjuan, Duan Zhongping, Li Xingwang, Liu Qingquan, Song Yuanlin, and Jia Zhenhua.
The scientificity of such trial grouping is controversial because the trial group also receives conventional treatment, and it will eventually be difficult to ascertain whether the trial drug has functions. This revision of the package insert also mentions the usage in "mild and ordinary" COVID-19, which may interfere with the "self-healing nature" of COVID-19 mentioned in earlier news reports. Plus, in the package insert of Lianhua Qingwen Capsules, "Patients should see a doctor if there is no remission of their symptoms 3 days after using the drug" conflicts with the newly revised "7-10 days", which may be because patients were already in the hospital during the trial. However, those need to be addressed for Lianhua Qingwen Capsules in the future.
From its receiving the pharmaceutical product approval number in 2009, entering the Chinese Pharmacopeia in 2010 to staying there in 2015, the path of Lianhua Qingwen Capsules has been smoother than many TCMs and even chemical drugs.
There is only Shijiazhuang Yiling Pharmaceutical Co., Ltd. manufacturing Lianhua Qingwen Capsules, whose stock price has enjoyed large increases recently, reaching up to 10.01% on Apr. 13. This approval on Apr. 12 is expected to lead to another increase.
The slight quieting of the epidemic in China may be not great for Lianhua Qingwen Capsules to achieve big sales, however, the drug is likely to be included in the reserve program as one of the few pharmaceutical products permitted to revise the package insert. Whether the overseas sales of Lianhua Qingwen Capsules can be accelerated due to the raging epidemic overseas still depends more on the clinical data.
The prices of many TCM decoction pieces in Lianhua Qingwen Capsules may rise correspondingly.
Lianhua Qingwen Capsules
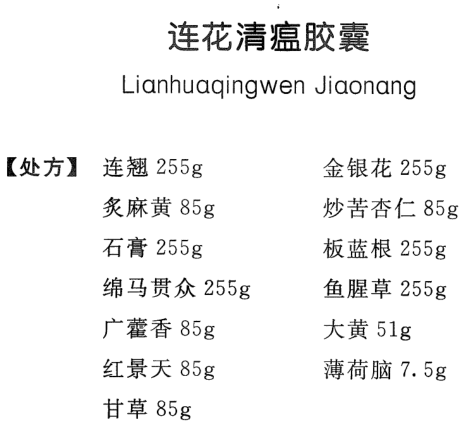
[Prescription]
Fructus forsythiae: 255g
Honeysuckle: 255g
Honey-fried herba ephedra: 85g
Stir-fried semen armeniacae amarum: 85g
Gypsum: 255g
Radix isatidis: 255g
Rhizoma dryopteris crassirhizomae: 255g
Herba houttuyniae: 255g
Pogostemon cablin: 85g
Rheum officinale: 51g
Rhodiola rosea: 85g
Menthol crystal: 7.5g
Liquorice: 85g
Unlike chemical drugs with technical barriers, people, after knowing the efficacy of the Chinese patent medicine, are highly likely to purchase TCM decoction pieces themselves in case of need. Pharmaceutical practitioners shall pay attention to avoiding blind drug use by the public. The approval of Lianhua Qingwen Capsules this time will induce the volatility of related products and is hoped to facilitate control over the epidemic.
References:
https://www.drugfuture.com/standard/search.aspx
http://www.chictr.org.cn/showproj.aspx?proj=48889
https://drugs.dxy.cn/
Chinese Pharmacopoeia
-----------------------------------------------------------------------
Editor's Note:
To become a freelance writer of En-CPhI.CN,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@ubmsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


