PharmaSources/CaicaiMarch 31, 2020
Tag: PARP inhibitor , Zai Lab , Niraparib , 2019
According to the 2019 results and company progress released by Zai Lab on Mar. 19, the total sales revenue of niraparib (trade name: Zejula) reached USD6.60 million (around RMB46.90 million) in Hong Kong, S.A.R., China and Macau, S.A.R., China region of China in 2019. Niraparib has been approved in Dec. 2019 in Mainland China and its sales revenue is expected to largely increase in 2020.
As a highly potent and selective poly (ADP-ribose) polymerase (PARP)-1/2 inhibitor, niraparib can bind to PARP and inhibit the dissociation of PARP from DNA-PARP compound to block subsequent DNA repair and play the anti-tumor role.
R&D process of niraparib:
Excellent clinical effects
The large-scale Phase 3 ENGOT-OV16/NOVA Clinical Trial was conducted for niraparib and recruited both patients with BRCA mutations and patients without BRCA mutations.
Clinical trial design: 553 patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who were in response to platinum-based treatment were recruited, including 203 with BRCA mutations and 350 without BRCA mutations; they separately received niraparib or placebo treatment according to the ratio of 2:1, with the starting dose of niraparib being 300mg/day.
According to the clinical data:
In patients with gBRCA (germline BRCA) mutations: The median progression-free survival (PFS) of patients in the niraparib group reached up to 21 months, while that of patients in the placebo group reached 5.5 months, meaning that the maintenance treatment with niraparib could reduce the disease progression or death risk by 73%;
In patients without gBRCA mutations but whose tumors tested positive for homologous recombination deficiency (HRD): The median PFS of patients in the niraparib group reached 12.9 months, while that of patients in the placebo group reached 3.8 months, meaning that the maintenance treatment with niraparib could reduce the disease progression or death risk by 55%;
In patients without gBRCA mutations and whose tumors tested negative for HRD: The median PFS of patients in the niraparib group reached 9.3 months, while that of patients in the placebo group reached 3.9 months. See the following figure for the details:
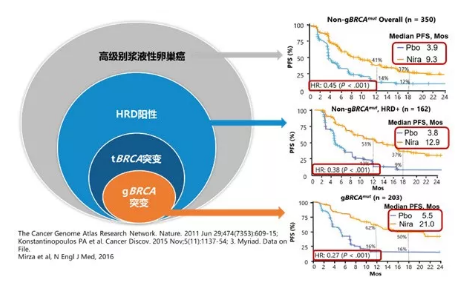
高级别浆液性卵巢瘤 | High grade serous ovarian cancer |
HRD阳性 | HRD positive |
tBRCA突变 | tBRCA mutation |
gBRCA突变 | gBRCA mutation |
We can see from the above clinical data that niraparib could significantly prolong patients’ PFS as a maintenance treatment no matter in patients with or without gBRCA mutations. Based on this, patients may consider not taking gene detection, however, different gene types correspond to different effects, and gene detection can help doctors or patients estimate the effects. After all, the drug can have better effects in patients with gBRCA mutations.
A total of 4 PARP inhibitors are marketed in the world
A total of 4 PARP inhibitors are marketed in the world, separately olaparib, rucaparib, niraparib, and talazoparib; wherein, olaparib is marketed in Mainland China, and niraparib is marketed in Hong Kong, S.A.R., China Region of ChinaRegion of China.
Pharmaceutical product name | Trade name | Company | Approval time and indication |
Olaparib | Lynparza | AstraZeneca | Dec. 2014: Approved by the FDA and EMA to treat patients with BRCA-mutated advanced ovarian cancer, who have been treated with three or more prior lines of chemotherapy; Sep. 2017 and May 2018: Separately approved by the FDA and EMA as a second-line maintenance treatment of adult patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in response to platinum-based drugs; Jan. 2018: Approved by the FDA to treat patients with BRCA-mutated HER2-negative metastatic breast cancer; Aug. 2018: Approved by the NMPA as a maintenance treatment of platinum-sensitive relapsed ovarian cancer; Dec. 2018: Granted by the FDA the orphan drug designation for the treatment of pancreatic cancer |
Rucaparib | Rubraca | Clovis | Dec. 2016: Approved by the FDA as a third-line treatment of patients with gBRCA-mutated ovarian cancer; Apr. 2018: Approved by the FDA for the maintenance treatment of adult patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in a complete or partial response to platinum-based chemotherapy |
Niraparib | Zejula | GSK, Zai Lab | Mar. 2017: Approved by the FDA for the maintenance treatment of female patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer (the first FDA-approved PARP inhibitor that can be used for treatment without the detection for BRCA mutation or other biomarkers and applies to a broader population; it is the world’s first PARP inhibitor for the second-line treatment of ovarian cancer); Oct. 2018: Approved in Hong Kong, S.A.R., China Region of China for the treatment of patients with platinum-sensitive relapsed high grade serous epithelial ovarian cancer, who are in a complete response (CR) or partial response (PR) to platinum-based chemotherapy; Dec. 2019: Approved for marketing in Mainland China |
Talazoparib | Talzenna | Medivation, Pfizer | Oct. 2018: Approved by the FDA for the treatment of adult patients with deleterious or suspected deleterious germline BRCA-mutated, HER2-negative locally advanced or metastatic breast cancer (it is called the "most potent" drug in the same class, however, it has not been formally marketed) |
(Source: FDA, EMA, NMPA )
Wherein, olaparib has entered the latest national reimbursement drug list (NRDL) of China, with the related agreement valid until Dec. 31, 2021.
Medical insurance region | No. | Pharmaceutical product name | Dosage form | Pharmaceutical product category | Medical insurance category | Supplementation | Remarks |
NRDL (2019) | 69 (negotiated) | Olaparib | Oral regular dosage form | Oncology drugs and immunomodulatory agents>Oncology drugs>Other oncology drugs>Other oncology drugs (XL>XL01>XL01X>XL01XX) | Category B | View | *; Only for platinum-sensitive recurrent epithelial ovarian, fallopian tube, or primary… |
(Source: db.yaozh.com)
Hengrui’s PARP inhibitor: fluzoparib has been applied for marketing
It is worth mentioning that Hengrui’s PARP inhibitor: fluzoparib has been applied for marketing and is expected to also be approved for marketing in 2020.
Acceptance No. | Pharmaceutical product name | Pharmaceutical product type | Application type | Registration class | Enterprise name | Acceptance date |
CYHS1900033 | Fluzoparib Capsules | Chemical drug | New drug | 1 | Jiangsu Hengrui Medicine Co., Ltd., Jiangsu Hengrui Medicine Co., Ltd. | Oct. 29, 2019 |
(Source: CDE)
Caicai, a Master of Pharmacy from Shanghai Jiaotong University, used to work in the Institute of Science and Technical Information. Currently as a practitioner in the drug surveillance system, she is good at interpreting industry regulations, pharmaceutical research developments, etc.
-----------------------------------------------------------------------
Editor's Note:
To become a freelance writer of En-CPhI.CN,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@ubmsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


