Tim FreemanMarch 25, 2020
Tag: API , Tim Freeman , Powder Flow , Dosing Performance
Dry Powder Inhalers (DPI) are used to deliver a controlled dose of active pharmaceutical ingredient (API) to the deep lung. Lactose is a commonly used as an excipient in this process, carrying the fine particles of the API from the capsule before being carried away as the API continues to the lungs. The coarser lactose is usually trapped in the throat and subsequently swallowed. The properties of the lactose will influence performance of the DPI, including during processes such as filling, dosing and drug release.
Identifying the properties of a powder or blend which enable the production of capsules of uniform mass allows new formulations to be optimised, without the significant financial and time implications associated with running samples through the process, and helps reduce the occurrence of poor dosage uniformity and high weight variation.

Obtaining Process Performance
Five lactose powders, with varying particle size distributions, were run through a dosator (Lab Dosator, 3P Innovation, Warwick, UK) using four different size outlets, (decreasing from Dosator 1 to Dosator 4). A dosed mass of 50mg with RSD<2% was targeted. RSD values for each lactose-dosator combination are shown in Table 1.
| D50 Particle Size Range, µm | Dosator 1 | Dosator 2 | Dosator 3 | Dosator 4 |
Lactose 1 | 180-250 | 2.27 | 2.4 | 2.06 | 0.89 |
Lactose 2 | 110-155 | 3.77 | 1.54 | 1 | 1.08 |
Lactose 3 | 70-110 | 1.84 | 0.85 | 0.79 | 0.56 |
Lactose 4 | 40-70 | 1.34 | 2.02 | 2.13 | 4.41 |
Lactose 5 | 4-11 | 3.76 | 7.05 | 7.59 | 8.32 |
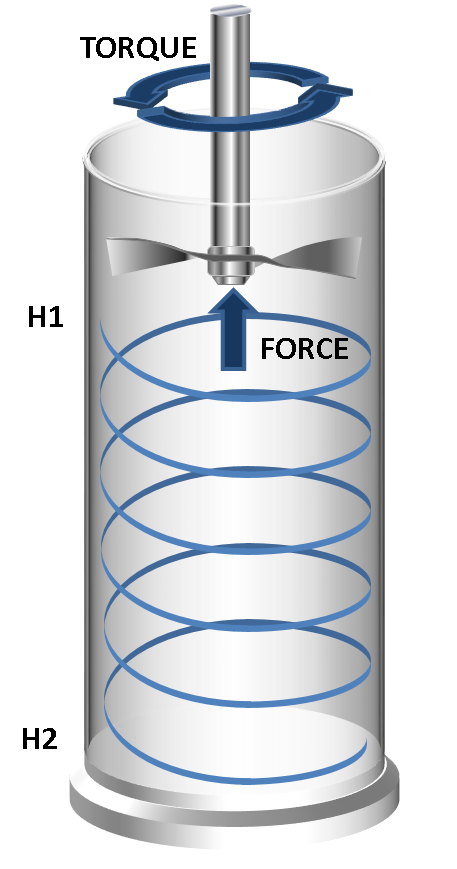
The FT4 Powder Rheometer® is a universal powder tester that provides automated, reliable and comprehensive measurement of bulk material characteristics. This information can be correlated with process experience to improve processing efficiency and aid quality control. Specialising in the measurement of dynamic flow properties, the FT4 also incorporates a Shear Cell, and the ability to measure bulk properties such as density, compressibility and permeability, enabling a comprehensive characterisation of the powder in a process relevant context.
Dynamic testing employs a patented measurement technique to determine a powder’s resistance to flow. A specially shaped blade traverses along a prescribed path through a precise volume of the powder. The force and the torque acting on the blade, as it moves axially and rotationally, are combined to generate a value for flow energy[1].

Aeration: Quantifying Inter-Particular Cohesion
The Aeration test evaluates the influence of increasing air content on the flow properties of a powder, and can be quantified by the Aerated Energy (AE) – the energy needed to establish a flow pattern while air is passed through the powder, at a given Air Velocity.
The data presented in Figure 1 and Table 2 shows that Lactose 1 is least sensitive to aeration, possibly due to a higher permeability. The profile of Lactose 5 shows the powder has a high level of cohesion, as shown by the higher Total Energy at 10mm/s Air Velocity. Conversely Lactoses 2-4 show a much lower level of cohesion. The differences between Lactoses 1-5 were most clearly seen in AE2, which was subsequently used in further analysis.
| AE2, mJ |
Lactose 1 | 2395 (±7%) |
Lactose 2 | 1500 (±1.7%) |
Lactose 3 | 725 (±6.1%) |
Lactose 4 | 180 (±3.5%) |
Lactose 5 | 410 (±5.6%) |
Table 2: Aerated Energy at 2mm/s Air Velocity (AE2)
Figure 1: Aeration test profiles showing varying sensitivities to aeration.
Specific Energy: Quantifying Inter-Particular Friction and Interlocking
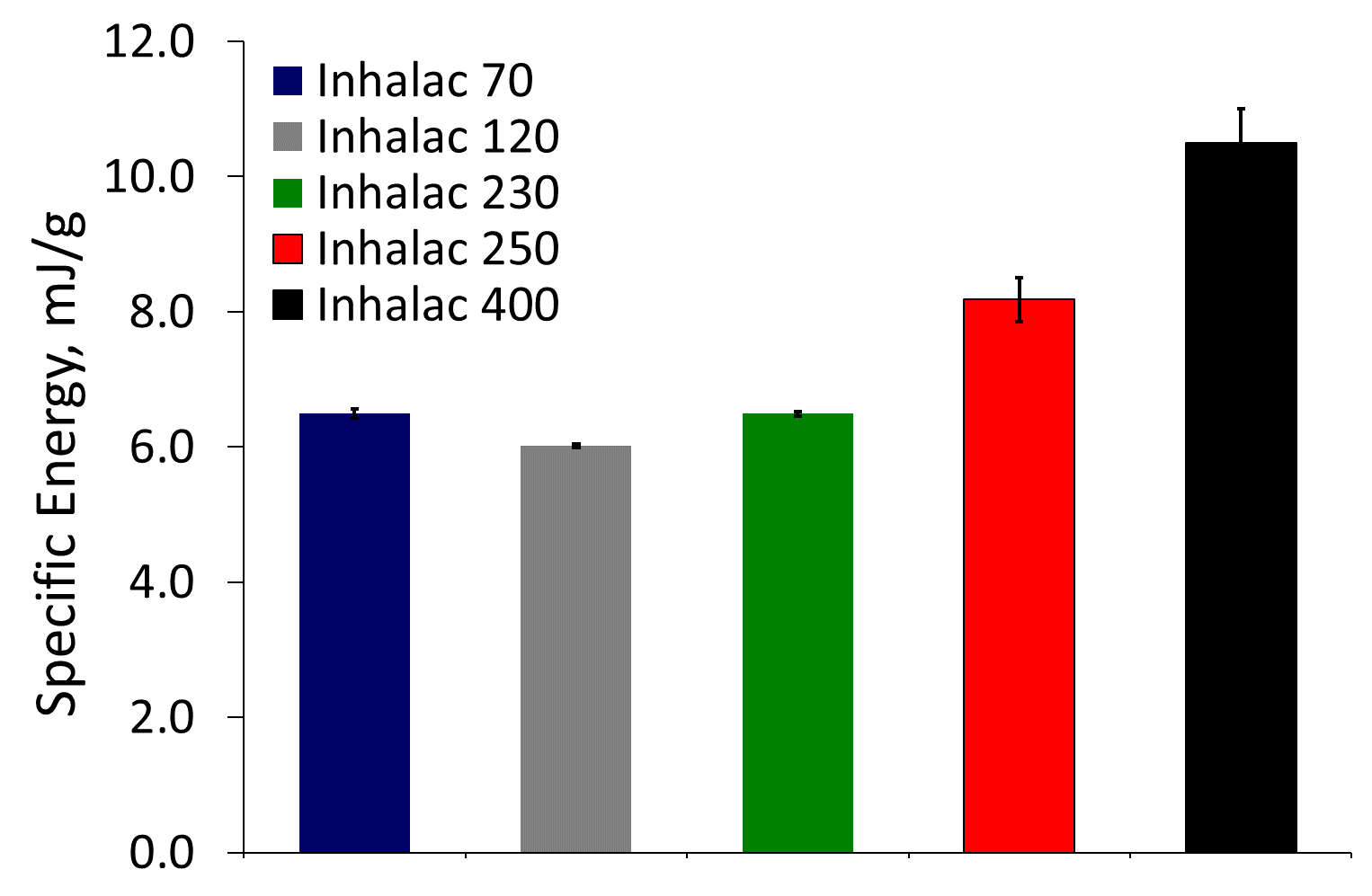
Specific Energy (SE) quantifies the resistance of particles moving relative to one another in an unconfined state. Particles of irregular shape and/or with rough surface texture will tend to lock together and form temporary mechanical bridges.
High SE values indicate greater levels of mechanical interlocking reducing the ability to flow in an unconfined environment which may be detrimental to dosing operations.

Figure 2 shows SE values for the five lactose powders. Lactose 5 has the highest SE, and Lactose 2 the lowest.
Rationalising Process Performance
The parameters introduced above provide a clear understanding of why the lactose powders perform differently in the various dosators.
Dosator 1:
► Lactose 3 and 4 exhibited acceptable performance (RSD<2%) in the largest dosator.
► Both powders generated lower AE2 values indicating greater sensitivity to aeration and lower inter-particular cohesion.
► Lactose 5 also generated an AE2 within this range, but had a much higher SE, indicating greater mechanical interlocking.
► For optimum performance in this dosator, compatible powders had a low AE2 and low SE.
Dosators 2 & 3:
► Optimal performance was observed with Lactose 2 and 3 in these two dosators.
► Both powders have low SE values indicating low inter-particular friction and mechanical interlocking.
► Lactose 1 and 4 exhibit near-acceptable performance, but have higher AE2 and SE respectively, likely inhibiting their performance. This demonstrates the need for a multivariate approach and how a single property is unlikely to dictate process behaviour.
► As with Dosator 1, optimum output is achieved with a low SE and a low AE2, however, a lower SE is required, and the Dosators are able to to tolerate a higher AE2.
Dosator 4:
► Lactose 1, 2 and 3 all exhibited acceptable performance in the smallest dosator.
► All three powders generate low SE values.
► Sensitivity to aeration appears to have little influence on performance in this dosator.
Conclusions
In order to ensure optimum dosing performance, the correct dosator must be chosen depending on the properties of the powder being used. This study demonstrates that dosator performance with different lactose powders changes depending on outlet size. In general, powders with lower SE and AE2 perform better in the process, but as dosator size decreases (from Dosator 1 to 4), the influence of AE2 decreases, whilst that of the SE increases. The larger dosator promotes a greater interaction with air at the outlet, reducing the impact of physical interactions and making aeration sensitivity more influential. By contrast, the smallest dosator has less opportunity for interaction with air and promotes physical interactions, making SE more influential in these cases.
The FT4’s multivariate approach is ideally suited to characterising the range of process-relevant powder properties that will influence the powder dosage in different dosators, by correlating measured powder properties with process performance. The correlations can be used to construct a design space of process-relevant powder properties that predicts the process behaviour, against which new formulations, incoming or outgoing batches, can be assessed.
For further information, please contact the Applications team on +44 (0)1684 851 551 or via support@freemantech.co.uk.
[1] Freeman R., Measuring the flow properties of consolidated, conditioned and aerated powders – A comparative study using a powder rheometer and a rotational shear cell. Powder Technology, 25-33, 174, 1-2, 2007
Author Biography

Tim Freeman, Managing Director, Freeman Technology
Tim Freeman is Managing Director of powder characterisation company Freeman Technology for whom he has worked since the late 1990s. He was instrumental in the design and continuing development of the FT4 Powder Rheometer® and the Uniaxial Powder Tester. Through his work with various professional bodies, and involvement in industry initiatives, Tim is an established contributor to wider developments in powder processing.
Tim has a degree in Mechatronics from the University of Sussex in the UK. He is a mentor on a number of project groups for the Engineering Research Center for Structured Organic Particulate Systems in the US and a frequent contributor to industry conferences in the area of powder characterisation and processing. A past Chair of the American Association of Pharmaceutical Scientists (AAPS) Process Analytical Technology Focus Group Tim is a member of the Editorial Advisory Board of Pharmaceutical Technology and features on the Industry Expert Panel in European Pharmaceutical Review magazine. Tim is also a committee member of the Particle Technology Special Interest Group at the Institute of Chemical Engineers, Vice-Chair of the D18.24 sub-committee on the Characterisation and Handling of Powders and Bulk Solids at ASTM and a member of the United States Pharmacopeial (USP) General Chapters Physical Analysis Expert Committee (GC-PA EC).
www.micromeritics.com.cn
info@freemantech.com.cn
-----------------------------------------------------------------------
Editor's Note:
En-CPhI.CN is a vertical B2B online trade platform serving the pharmaceutical industry,
for any copyright disputes involved in the reproduced articles,
please email: Julia.Zhang@ubmsinoexpo.com to motify or remove the content.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


