PharmaSources/CaicaiMarch 19, 2020
Nonalcoholic fatty liver disease (NAFLD) includes nonalcoholic fatty liver (NAFL), nonalcoholic steatohepatitis (NASH), and NASH-related liver cirrhosis; wherein, NASH refers to the hepatocyte inflammation that occurs on the basis of hepatocyte steatosis and is a severe type in NAFLD. NAFL, if left untreated, may develop to NASH and the latter may progress to liver cirrhosis. NAFLD is the most common cause of hepatocellular carcinoma (HCC) in the U.S. According to a study in the U.S., the NAFLD-related HCC accounts for 59% of all the HCC types.
NAFLD Progression
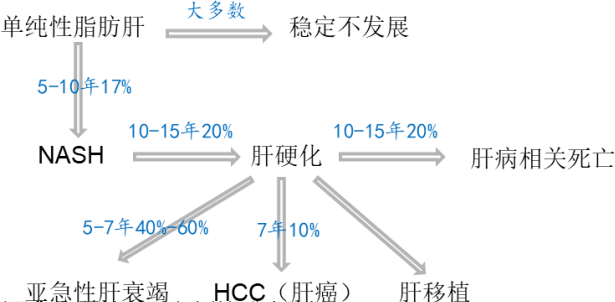
单纯性脂肪肝 | NAFL |
大多数 | Most patients |
稳定不发展 | Stable and not progressing |
5-10年17% | 17% of patients in 5-10 years |
10-15年20% | 20% of patients in 10-15 years |
肝硬化 | Liver cirrhosis |
肝病相关死亡 | Liver disease-related death |
5-7年40%-60% | 40-60% of patients in 5-7 years |
7年10% | 10% of patients in 7 years |
亚急性肝衰竭 | Subacute liver failure |
HCC(肝癌) | HCC (liver cancer) |
肝移植 | Liver transplantation |
NAFLD is the most common chronic liver disease in the world, with a worldwide incidence of 25% and an incidence of 20% in China. There are about 200-300 million NAFLD patients in China, with the number of NASH patients estimated to be more than 30 million. Obesity, type 2 diabetes, and hyperlipidemia and hypertension are the three major risk factors of NAFLD. The incidence of NAFLD in obese people is as high as 70% and the prevalence thereof has a rising trend as the number of people with obesity and hypertension, hyperglycemia and hyperlipidemia is growing.
NAFLD Prevalence
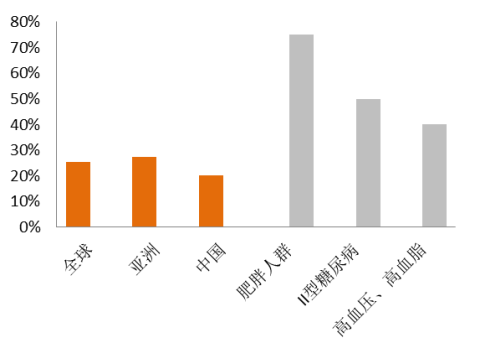
全球 | World |
亚洲 | Asia |
中国 | China |
肥胖人群 | Obese people |
II型糖尿病 | Patients with type 2 diabetes |
高血压、高血脂 | Patients with hyperlipidemia and hypertension |
As a metabolic syndrome, NASH often comes together with obesity and hypertension, hyperglycemia and hyperlipidemia, therefore, NASH treatment includes 4 aspects: basic treatment (changing the lifestyle), treatment against metabolic syndrome, treatment against liver injury, and liver transplantation. As the NASH patient group is huge and there is no marketed drug with definite efficacy in the world, the first breakthrough drug may become a blockbuster. Numerous pharmaceutical enterprises are working hard on the related drugs.
According to the forecast of EvaluatePharma, the global NASH drug market will reach USD40 billion by 2025. Such a huge market is in urgent need of blockbusters.
Indian Saroglitazar lifting the curtain
Saroglitazar of an Indian pharmaceutical enterprise has recently been approved by the Drug Controller General of India to become the world’s first drug approved to treat NASH to formally lift the curtain of the NASH drug market.
As a PPARα/γ agonist, Saroglitazar was marketed in India in Sep. 2013 for the treatment of dyslipidemia and hypertriglyceridemia in patients with type 2 diabetes not controlled by statins alone; the indication of the drug was approved to expand to the treatment of patients with type 2 diabetes in Jan. 2020.
The approval this time was based on the phase III clinical trial EVIDENCESII. Saroglitazar achieved positive results in NASH treatment in the said trial conducted in India. The observation period of the trial was 52 weeks, and the liver histology improvement of NASH patients was assessed through liver biopsy at the end of week 52. The trial successfully reached the primary endpoint and secondary endpoint.
Refer to "The World’s First Novel Drug against NASH First Approved to be Marketed in India" for the details.
Obeticholic acid with the fastest progress
2019 was definitely the most memorable year in the NASH/NAFLD drug R&D process, where many potential varieties to treat NASH that people were generally optimistic about were announced to reach the clinical endpoints in large-scale clinical studies and also many new varieties enter phase II/III clinical studies.
Wherein, obeticholic acid had the fastest progress.
According to the 18-month interim analysis results of the large-scale phase III clinical trial REGENERATE of obeticholic acid announced by Intercept in Feb. 2019, in the group medicated with 25mg dose, the proportion of patients with fibrosis improvement of more than 1 stage and with no worsening of NASH had statistical significance compared to the placebo group (23.1%vs.11.9%, p=0.0002). Among the patients with F1/F2/F3-stage liver fibrosis, in the group medicated with 25mg dose, the proportion of patients with fibrosis improvement of more than 1 stage and with no worsening of NASH also had statistical significance compared to the placebo group (21.0%vs.10.6%, p<0.0001); in terms of the indicator of NASH resolution with no worsening of liver fibrosis stage, the medicated group also had a statistically significant difference (14.9%vs.7.9%, p=0.0013) compared to the placebo group. However, obeticholic acid was not perfect: in terms of the side effects that occurred in the medicated group, the proportion of patients with pruritus in the high-dose group reached as high as 51%, and the incidence of moderate to severe pruritus reached as high as 28%; furthermore, 17% of patients in the high-dose group had an increase in LDL.
Based on the above clinical data, Intercept filed the NDA for the indication of treating NASH with obeticholic acid to the FDA in Sep. 2019, which received the priority review. Intercept announced in Jan. 2020 that the FDA had postponed the PDUFA date for 3 months from the original Mar. 26 to June 26.
NASH/NAFLD Drugs that have Entered Phase III Clinical Trials Overseas
Drug name | Developed by | Target | Mechanism of action | Clinical trial phase |
Obeticholic acid | Intercept | FXR agonist | Reduce liver fatty degeneration, improve insulin resistance and inhibit liver inflammation and fibrosis | Phase Ⅲ (with the NDA filed to the FDA) |
MGL-3196 | Madrigal | Thyroid hormone receptor beta agonist | Reduce the fat content of the liver | Phase Ⅲ |
GS-0976 | Gilead | Acetyl-CoA carboxylase inhibitor | Reduce the fat content of the liver and reduce fibrosis and liver biochemical marker levels | Phase Ⅲ |
Elafibranor | Genfit&Tens | PPARα/δ agonist | Enhance fatty acid oxidation and insulin sensitivity, improve lipid profile and resist inflammation and fibrosis | Phase Ⅲ |
Cenicriviroc | Allergan | Chemokine receptor CCR2/5 antagonist | Improve liver fibrosis | Phase Ⅲ |
(Organized according to public data. Any supplementation will be welcomed.)
NASH drug R&D in China using the "buying" method
Numerous Chinese pharmaceutical enterprises and health care products suppliers are also actively getting involved in the NASH drug area, with the candidate drugs leading in the progress mostly developed in cooperation with overseas companies.
In Feb. 2019, Ascletis Pharma reached an agreement with 3-VBiosciences in connection with the strategic cooperation in and exclusive development of TVB-2640 in Greater China. TVB-2640 (Ascletis’ code: ASC40) is a fatty acid synthase inhibitor and a candidate drug to treat NASH. It is currently in clinical phase II.
Terns signed an agreement with Genfit in June 2019, where Terns would contribute USD228 million upfront payment and milestone payment to obtain the exclusive development, registration and commercialization rights and benefits of Elafibranor in indications such as NASH and PBC. The drug is currently in clinical phase III.
Latest Study Progress of NASH/NAFLD Drugs in China
Drug | Manufacturer | Target | Mechanism of action | Clinical trial phase |
Elafibranor | Terns &Genfit | PPARα/δ agonist | Enhance fatty acid oxidation and insulin sensitivity, improve lipid profile and resist inflammation and fibrosis | Phase Ⅲ (overseas) |
ASC40 | Ascletis &3-V | Fatty acid synthase (FASN) inhibitor | Inhibit fat synthesis and resist liver fibrosis | Phase Ⅱ (global multicenter) |
Namodenoson (CF102) | China Medical System&Can-Fite | A3 adenosine receptor agonist | Resist inflammation | Phase Ⅱ (overseas) |
HTD1801 | Hightide | Multifunctional small molecule drug | Improve liver histological characteristics, reduce inflammation and lower liver cholesterol and triglyceride levels | Phase Ⅱ (the U.S.) |
ZSP1601 | Zhongsheng Pharmaceutical | N/A | Resist liver fibrosis | Phase Ib/IIa |
(Organized according to public data. Any supplementation will be welcomed.)
Furthermore, Chia Tai Tianqing, HEC Pharm, and Zhongsheng Pharmaceutical, etc. have also got involved in the related drugs, however, all of them are in clinical phase I.
Caicai, a Master of Pharmacy from Shanghai Jiaotong University, used to work in the Institute of Science and Technical Information. Currently as a practitioner in the drug surveillance system, she is good at interpreting industry regulations, pharmaceutical research developments, etc.
-----------------------------------------------------------------------
Editor's Note:
En-CPhI.CN is a vertical B2B online trade platform serving the pharmaceutical industry,
for any copyright disputes involved in the articles,
please email: Julia.Zhang@imsinoexpo.com to motify or remove the content.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


