PharmaSources/Zhulikou431March 20, 2020
Tag: Clinical Trial , COVID-19 , Sivelestat Sodium
In the notice on pharmaceutical product approval released on the website of the National Medical Products Administration of China (NMPA) on Mar. 12, 2020, the Sivelestat Sodium Hydrate for Injection developed by Shanghai Huilun Jiangsu Pharmaceutical (“Shanghai Huilun”) has attracted the attention of the industry, and the stock price of Xintian Pharmaceutical that has a stake in the company hit the daily upper limit along with the notice. The NMPA has so far urgently approved the clinical trials of 5 products against the COVID-19 to better prevent and control it, including sivelestat sodium hydrate, favipiravir, remdesivir, and BDB-001 injection, etc.
According to the information on the NMPA website and the information of Shanghai Huilun, the marketing application for Sivelestat Sodium Hydrate for Injection (CYHS2000102) has been approved by the NMPA. According to Xintian Pharmaceutical, Shanghai Huilun has filed the marketing application for Sivelestat Sodium Hydrate for Injection according to Class 3 generic drug in Feb. 2020. Sivelestat sodium hydrate has been proved its effects in many phases of clinical trials, and the company has obtained the GMP certificate, which means that it has reached the commercial production conditions and can mass-produce the drug after receiving the approval.
Part I: Product development background information
It is verified that sivelestat sodium hydrate was first developed by Ono Pharmaceutical. It is a competitive inhibitor of neutrophil elastase (NE). The neutrophil and the elastase released thereby play an important role in the pathogenesis of acute lung injury (ALI). Approved for marketing in Japan in 2002, the pharmaceutical product is used to treat ALI or acute respiratory distress syndrome (ARDS) associated with systemic inflammatory response syndrome (SIRS). It is currently the world’s only ALI therapeutic drug approved for marketing.
Sivelestat Sodium Hydrate for Injection was used as a candidate drug for SARS in 2003 after the SARS epidemic broke out in China and even the world, and it became the first SARS therapeutic drug that entered the clinical trial in China after the former China Food and Drug Administration (CFDA) launched the fast track. Many pharmaceutical companies obtained the clinical trial approval for Sivelestat Sodium Hydrate for Injection back then, however, most pharmaceutical companies did not proceed with the related clinical trial of this drug thereafter as the SARS epidemic was over. It is verified that Shanghai Huilun filed the marketing application for Sivelestat Sodium Hydrate for Injection in 2014, however, the well-known “July 22 clinical trial data checking storm event” that occurred in 2015 also affected Sivelestat Sodium Hydrate for Injection of Shanghai Huilun.
Part II: The registration progress and application situation of sivelestat sodium hydrate
According to my queries on DXY Insight database, 46 enterprises have approved and started the R&D applications for sivelestat sodium hydrate project by Feb. 14, 2020.
Analysis: Only Shanghai Huilun has held on to the last to receive the marketing approval. The marketing of sivelestat sodium hydrate of Shanghai Huilun was delayed as affected by the checking storm in 2015, however, the company eventually succeeded after follow-up R&D efforts. Predictably, more pharmaceutical companies that research deep into the project will actively get involved with the breakthrough of Shanghai Huilun and the worsening of the COVID-19 epidemic.
Part III: Clinical trial progress of sivelestat sodium hydrate
According to my queries on DXY Insight database, 5 clinical trials of sivelestat sodium hydrate have been launched and completed in China. See the following table for the specific data:
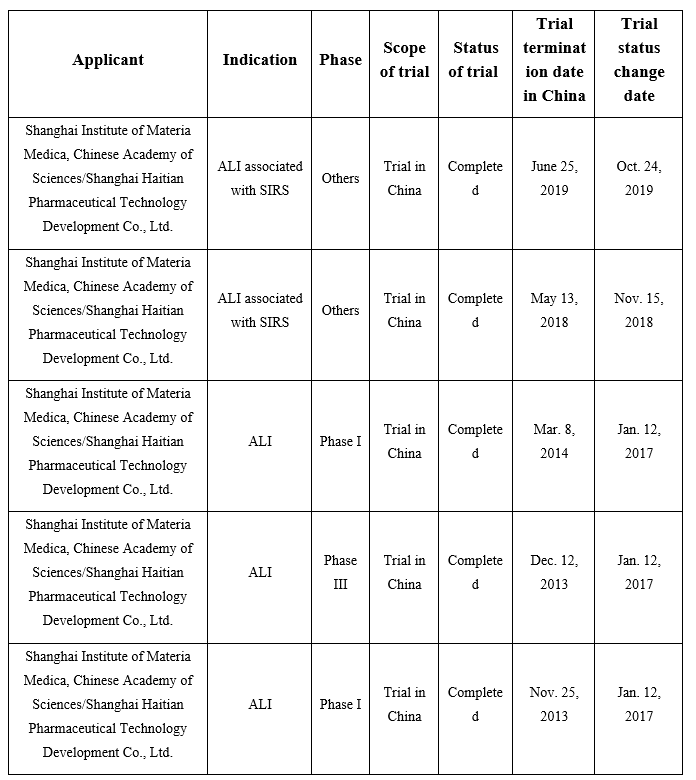
Analysis: The applicant of clinical trials in the above table is Shanghai Haitian Pharmaceutical Technology Development Co., Ltd. It’s verified that Shanghai Haitian is a wholly-owned subsidiary established by Guiyang Xintian Pharmaceutical Co., Ltd. in Shanghai, being the R&D platform and academic marketing window of Xintian Pharmaceutical, while Xintian Pharmaceutical has a stake in Shanghai Huilun.
Part IV: Information of Shanghai Huilun
According to the NMPA database, the scope of production of Shanghai Huilun includes Building 28, No. 801, Jiankang Avenue, Taizhou City: lyophilized powder for injection, tablets, hard capsules, granules, small-volume injections (penicillin bottles); APIs (entecavir, esomeprazole magnesium, esomeprazole sodium, sivelestat sodium hydrate, tenofovir disoproxil fumarate, levofolinate, palonosetron hydrochloride, rivaroxaban, ticagrelor, folinic acid, calcium levofolinate, dexlansoprazole, avanafil, tenofovir alafenamide fumarate, sugammadex sodium, silodosin, empagliflozin), (oncology drugs: azacitidine, temozolomide, huinuopali, yinuopali).
Determined according to the above information, Shanghai Huilun has the required qualification and commercial production capacity. It can rapidly start the commercial production of sivelestat sodium hydrate after obtaining the NMPA’s approval.
Conclusion
According to the above information and data, Shanghai Huilun’s obtaining of the marketing approval for sivelestat sodium hydrate will enable it to provide more choice of drugs against the COVID-19. And other companies with technologies in similar products and having the ability to apply for production will also actively grasp the chance of this epidemic to rapidly file the applications.
With the injection consistency evaluation being conducted in China, subsequent applications involving sivelestat sodium hydrate may all need to be reviewed according to the injection consistency evaluation requirements.
Note: This article does not constitute any value judgement or investment advice.
References
1-Information on NMPA website
2-CDE database
3-DXY Insight database
4-Information on FDA website
Zhulikou431, as a senior engineer, PDA member, ISPE member, ECA member, PQRI member, senior aseptic GMP expert, has deep knowledge in aseptic process development and verification, drug development and registration, CTD document writing and review, regulatory audit, international certification, international registration , quality system construction and maintenance, as well as sterile inspection, environmental monitoring and other fields. In recent years, he has focused on the analysis of trends in the macro pharmaceutical field and the risk management of pharmaceutical enterprise mergers and acquisitions projects.
-----------------------------------------------------------------------
Editor's Note:
En-CPhI.CN is a vertical B2B online trade platform serving the pharmaceutical industry,
for any copyright disputes involved in the articles,
please email: Julia.Zhang@imsinoexpo.com to motify or remove the content.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


