Janssen has announced that the European Medicines Agency (EMA) has granted both PRIME (PRIority MEdicines) and Advanced Therapy Medicinal Product (ATMP) designations to its adeno-associated virus (AAV)-RPGR gene therapy product, for the treatment of inherited retinal disease X-linked retinitis pigmentosa (XLRP).
The novel AAV-RPGR drug, which is being jointly developed with the Johnson & Johnson company and MeiraGTx Holdings, also received Fast Track designation from the US Food and Drug Administration (FDA) and Orphan designations from the FDA and the EMA.
Janssen confirmed that the decision was made based on data from an ongoing Phase I/II clinical trial, which has been running since 2017, and that the designation makes the drug the only XLRP treatment in development to be awarded PRIME designation.
"The PRIME and ATMP designations for our gene therapy asset are important achievements for Janssen's growing retinal portfolio and bring us one step closer to delivering a transformational therapy to European patients living with X-linked retinitis pigmentosa," said James List, global therapeutic area head, Cardiovascular & Metabolism, Janssen.
He continued, "We look forward to partnering with the EMA and appreciate their dedication to patients underserved with current options."
Currently there are no approved treatments for XLRP, and Janssen's AAV-RPGR gene therapy is designed to treat the most common form of the disease, caused by mutations in the RPGR gene, by slowing the retinal degeneration and preserving visual function.
PRIME is a designation awarded to increase interactions, optimise development plans and accelerate innovative treatments where there is unmet medical need, and ATMP status is granted to medicines that are based on genes, tissues or cells and can offer groundbreaking opportunities for the treatment of disease.
Register as Visitor to CPhI China 2020!

-----------------------------------------------------------------------
Editor's Note:
En-CPhI.CN is a vertical B2B online trade platform serving the pharmaceutical industry,
for any copyright disputes involved in the articles,
please email: Julia.Zhang@imsinoexpo.com to motify or remove the content.





 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story

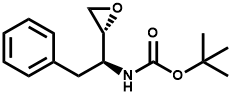
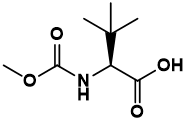

















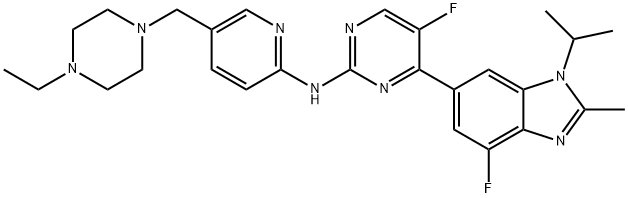
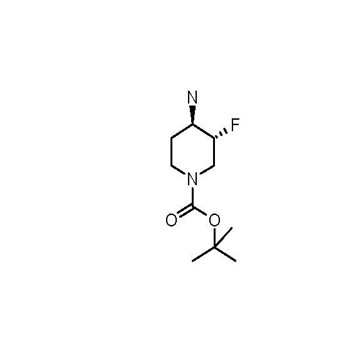


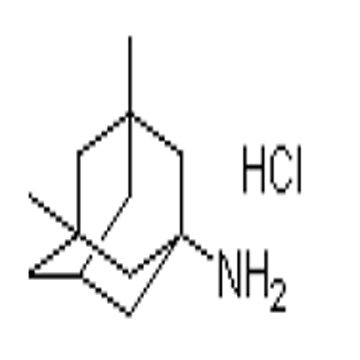







 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








