Tim FreemanFebruary 21, 2020
Tag: Freeman Technology , Powder Flow , tablet manufacture , Powder testing techniques
Related Reading: Powder Flow: Powder testing techniques for tablet manufacture (1)
Choosing the best powder characterization techniques
The example of the tablet press shows how important it can be to understand how the powder will behave under very different conditions, thereby highlighting the limitations of applying a single powder testing technique. Historically the pharmaceutical industry has relied on parameters such as Hausner Ratio and Carr’s Index, both derived from tapped density techniques, and more recently shear testing. These approaches do have some relevance when trying to access information to support optimization of the press.
Shear testing, for example, was developed, and remains widely used, for the design and troubleshooting of hoppers. It provides valuable shear strength data that is relevant to design and operation of the feed hopper, and also to the compression stage where applied stresses are even higher. However, as the data in Figure 2 shows, shear testing may be less useful than other techniques for generating data to support other parts of the tablet manufacturing process, where applied stresses are far lower.
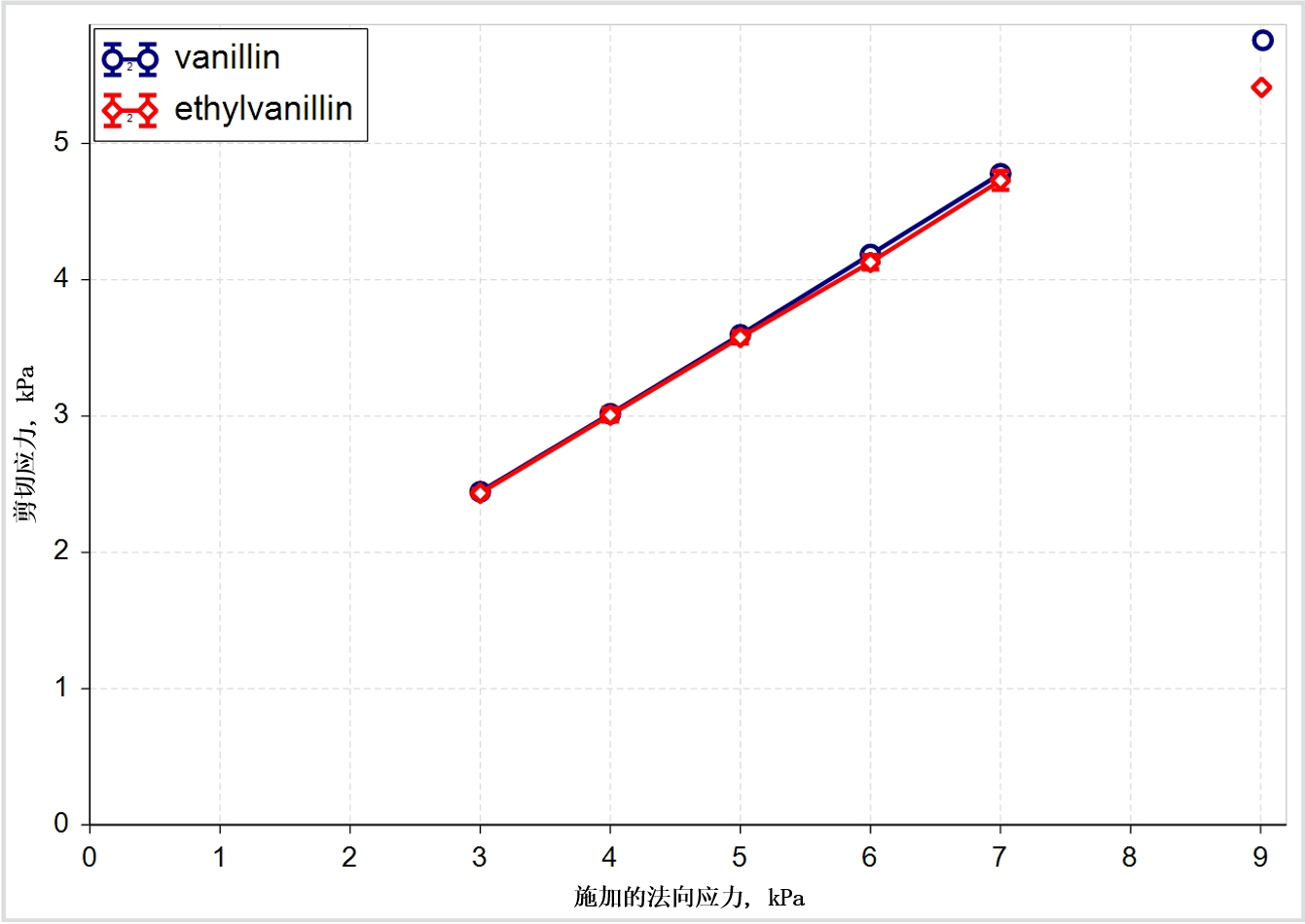
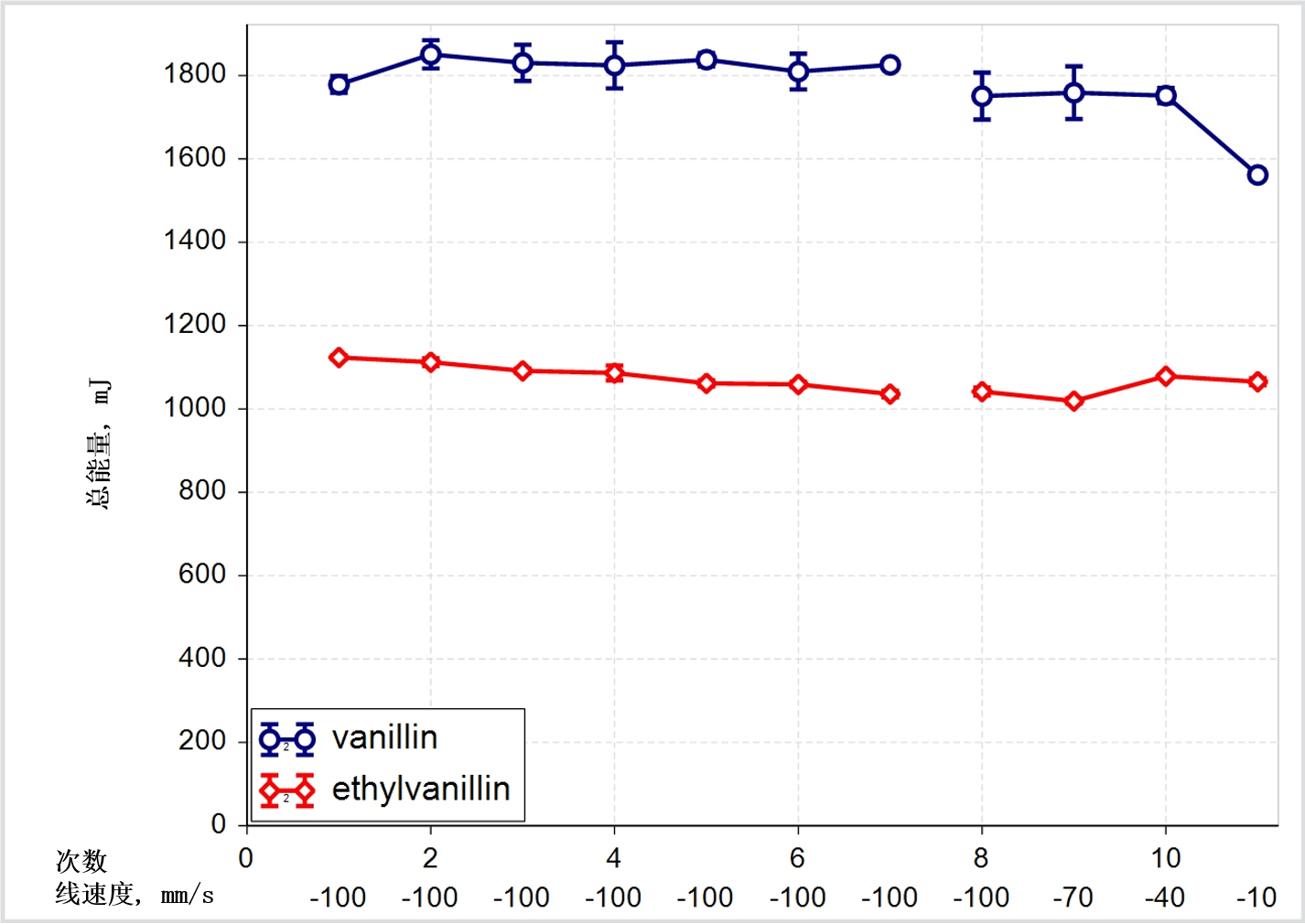
Figure 2(a) + 2(b): Shear data classifies these two excipients as closely similar while flow energy measurements show that in certain circumstances they can behave quite differently
The shear data presented for vanillin and ethylvanillin classifies them as closely similar but the dynamic flow energy measurements tell a different story, suggesting that the materials can behave differently in certain circumstances. Flow energies are generated by measuring the axial and rotational forces acting on a blade as it rotates through a powder sample3. Downward rotation of the blade imposes a forcing, bulldozing action while an upward traverse measures a flow energy more closely associated with gravity induced flow.
Here then the data suggest that while these two materials may behave similarly in the hopper, and in terms of how they cohere in the finished tablet, they will behave very differently in the feed frame. The ease with which the materials flow into the feed frame, and subsequently into the die, as well as their response to a range of blade designs, are all likely to be different.
This comparison raises the question of how useful other techniques that are routinely used to investigate and compare flow might be for this application. Figure 3 contrasts tapped density measurements with flow energy data.
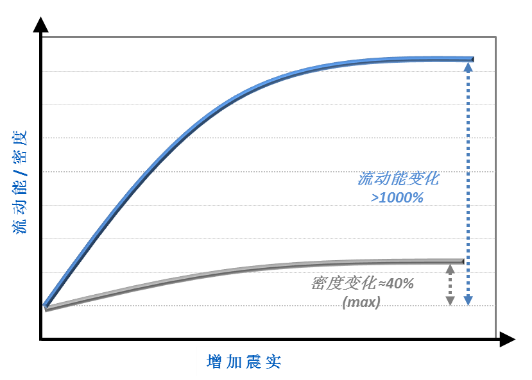
Figure 3: For this system tapped density data are far less sensitive than flow energy measurements in the detection of changes in powder flowability
These results show that although the tapped density measurements do detect changes that indicate differences in powder properties, they are far less sensitive than dynamic techniques in specifically measuring flow properties. Tapped density measurements could therefore fail to detect variability in a material that would go on to impact flow behaviour in the tablet press.
Looking across the tablet making process in its entirety highlights the range of properties that could advantageously be measured to optimize performance at each step. These include:
Together these measurements form a database of properties that can be used to tightly define an optimal blend specification. Furthermore, such data would support investigation into which parameters in upstream processes ultimately dictate performance in the press and the quality of the final product. Such strategies are highly beneficial in pushing towards more efficient manufacture on the basis of secure knowledge.
In conclusion
Within the pharmaceutical industry there is increasing emphasis on processing efficiency and more effectively controlled manufacture. In production it is impossible to eliminate all the possible sources of variability but it is feasible to compensate for them and control their impact. This relies on identifying, measuring and controlling those parameters that define processing efficiency and the quality of the finished product.
In solid dosage manufacture achieving this goal requires a relevant and sensitive powder testing toolkit capable of reliably generating parameters that can be directly correlated with process performance. Key to this is the ability of a technique to effectively simulate the specific processing environment, which, as an analysis of tableting reveals, can vary considerably. Within this context instruments that combine established techniques such as shear and bulk property testing, with newer methodologies with proven value such as dynamic testing, are especially valuable. By presenting a selection of techniques such systems allow the user to closely tailor testing to the specific application and access the best information for process design, optimisation and control.
Related Reading:
Powder Flow: Powder testing techniques for tablet manufacture (1)
References:
[1] ‘Process Robustness’ PQRI white paper. Available for download at: http://www.pqri.org/pdfs/whitePapers/process_robustness_White_Paper-final_draft_of_10_05.pdf
[2] Carlson, G and Hancock, B ‘Development of a material sparing method to determine powder permeability to air’ Delivered at AAPS 2008.
[3] Freeman R. “Measuring the flow properties of consolidated, conditioned and aerated powders — A comparative study using a powder rheometer and a rotational shear cell”, Powder Technology 174 (2007) 25–33.
Author Biography

Tim Freeman, Managing Director, Freeman Technology
Tim Freeman is Managing Director of powder characterisation company Freeman Technology for whom he has worked since the late 1990s. He was instrumental in the design and continuing development of the FT4 Powder Rheometer® and the Uniaxial Powder Tester. Through his work with various professional bodies, and involvement in industry initiatives, Tim is an established contributor to wider developments in powder processing.
Tim has a degree in Mechatronics from the University of Sussex in the UK. He is a mentor on a number of project groups for the Engineering Research Center for Structured Organic Particulate Systems in the US and a frequent contributor to industry conferences in the area of powder characterisation and processing. A past Chair of the American Association of Pharmaceutical Scientists (AAPS) Process Analytical Technology Focus Group Tim is a member of the Editorial Advisory Board of Pharmaceutical Technology and features on the Industry Expert Panel in European Pharmaceutical Review magazine. Tim is also a committee member of the Particle Technology Special Interest Group at the Institute of Chemical Engineers, Vice-Chair of the D18.24 sub-committee on the Characterisation and Handling of Powders and Bulk Solids at ASTM and a member of the United States Pharmacopeial (USP) General Chapters Physical Analysis Expert Committee (GC-PA EC).
www.micromeritics.com.cn
info@freemantech.com.cn
Register as Visitor to CPhI China 2020!

-----------------------------------------------------------------------
Editor's Note:
En-CPhI.CN is a vertical B2B online trade platform serving the pharmaceutical industry,
for any copyright disputes involved in the reproduced articles,
please email: Julia.Zhang@ubmsinoexpo.com to motify or remove the content.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


