PharmaSources/1°CDecember 05, 2019
Tag: biosimilar , autoimmune , adalimumab
Adalimumab has been "active" recently. Multiple adalimumab biosimilars are entering the harvest period in China following the arrival of China’s biosimilar era starting with Henlius’ Hanlikang (rituximab)!
AbbVie announced on Nov. 4, 2019 that Humira (adalimumab) has been approved for polyarticular juvenile idiopathic arthritis (pJIA) which is its 4th indication approved in China following rheumatoid arthritis, ankylosing spondylitis, and psoriasis.
Bio-Thera’s adalimumab biosimilar QLETLI (BAT1406) has been approved for marketing on Nov. 7, 2019, which is the first adalimumab biosimilar approved for marketing in China.
Junshi Biosciences announced on Nov. 8, 2019 that the NMPA has accepted the marketing application (No.: CXSS1900041) of the adalimumab biosimilar UBP1211 independently developed by it, which will be the 5th adalimumab biosimilar approved in China. If you are looking for hospital supply companies who could supply biosimilar products, Pharmasources would be your best choice.
What is more worth noting is that the marketing applications for many indications of AbbVie’s original drug Humira (adalimumab) are still at the review stage. Besides the updates on the review of the original drug and biosimilars of adalimumab, J & J’s ustekinumab and Novartis’ Cosentyx (secukinumab) were separately approved in 2017 and 2019 in China, and J & J’s guselkumab is expected to be also approved in 2020 in China.
Then, which will win the competition in the Chinese autoimmune disease drug market after super blockbuster drugs for TNFα, IL-17, and IL-23 have entered China and innovative drugs and biosimilars have been marketed in China in succession?
China’s national medical insurance is gradually becoming the biggest buyer in the Chinese pharmaceutical market. Pricing is a key factor in the Chinese autoimmune disease drug market, and for now, only autoimmune disease drugs that are guaranteed supply and priced appropriately can stand out. Yisaipu and Humira are good examples thereof.
I. Global autoimmune disease drug market: a succession of waves
The global autoimmune disease drug market is big. As far as some autoimmune disease drugs are concerned, the market size of the original drugs has reached USD55 billion. We can know by combining the market and clinical data of innovative drugs and biosimilars, sales of anti-TNFa drugs represented by Humira have peaked, and the market of super blockbusters in this area will be gradually nibbled by biosimilars or the new-generation breakthrough drugs in the next 5-10 years, with the latter represented by the anti-IL-17 drug Cosentyx (secukinumab), anti-IL-23 monoclonal antibody Tremfya (guselkumab), and JAK small molecule drugs.
Market Data of Main Autoimmune Disease Drug Products
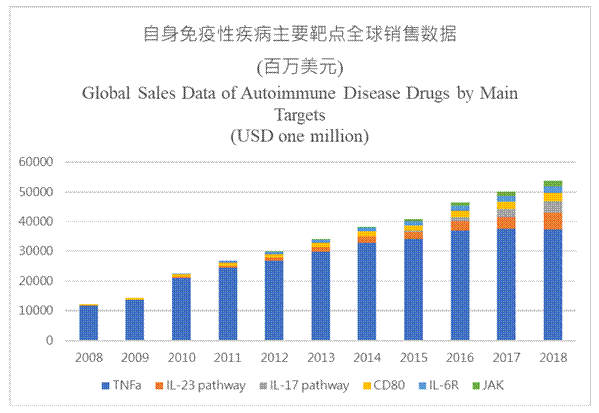
Source: Annual reports of the companies
Forecast of the market performance of main drugs
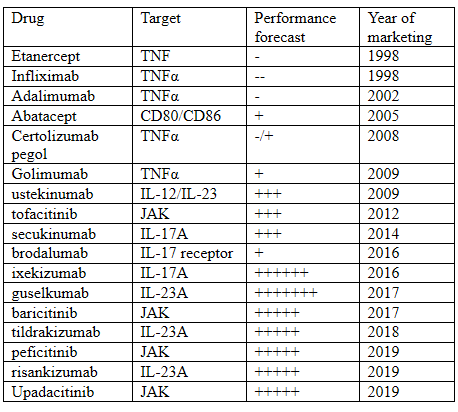
Growth: "+"10-20%; "++"20-30%; "+++"30-40%; "++++"40-50%;
"+++++">50%
See other analyses in the article A Review of Star Pharmaceutical Products in the Financial Reports of Pharmaceutical Enterprises in 2019: Industry Trends (Part One).
II. Chinese autoimmune disease drug market: early marketing and low prices will lead to good markets
The Chinese autoimmune disease drug market is vastly different from the global market pattern, and the market size of the macromolecular drugs for autoimmune diseases in China is only RMB1.6 billion, with the best-selling product being 3SBio’s Yisaipu. Targets and annual costs of the drug molecules are summarized in the following table:
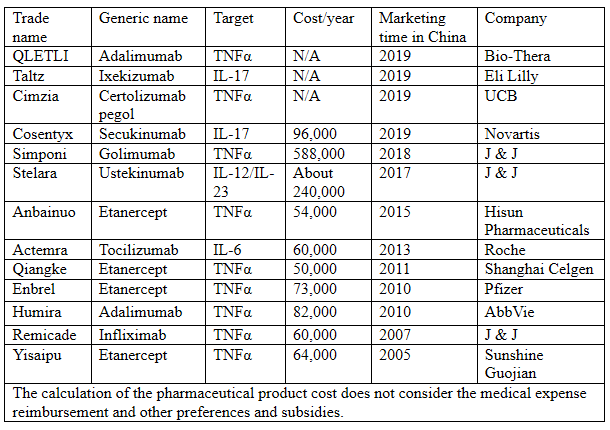
Tocilizumab: Adjusted from RMB1,925.00/piece to RMB830.00/piece, -57%, 60kg, about RMB60,000/year
Humira: Adjusted from RMB7,608.38/piece to RMB3,160/piece, -58%, RMB82,000/year
Enbrel: Adjusted from RMB2,400/piece to RMB699/piece, -70%, RMB73,000/year;
There have been 13 macromolecular drugs for autoimmune diseases approved for marketing in China, as shown in the above table, including the old etanercept, infliximab and adalimumab, etc., and the new ustekinumab and secukinumab.
According to the official data of 3SBio, its Yisaipu was marketed early and has a low price in the area of China and still accounts for half of the market.
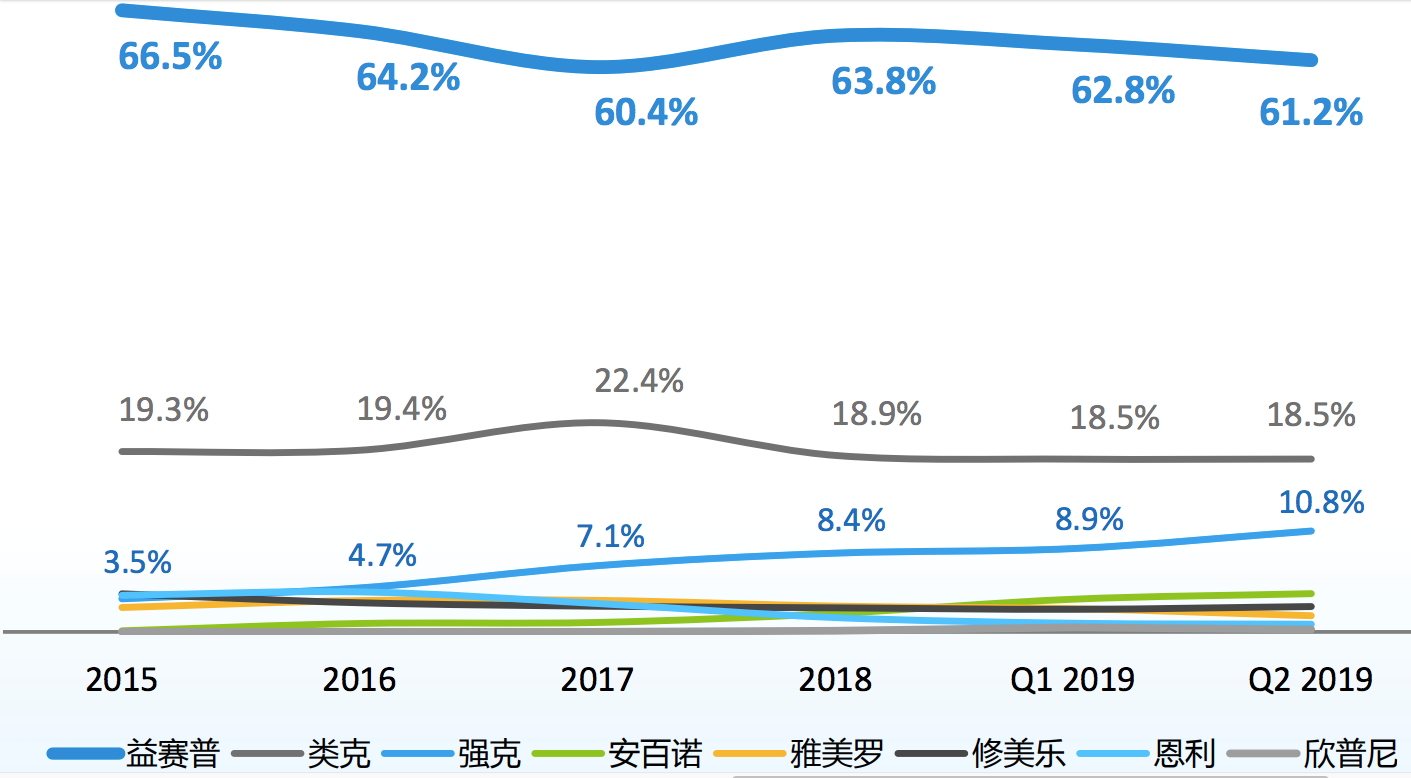
Source: 3SBio, IMS
3 etanercept biosimilars, tocilizumab, and golimumab have now been included in China’s national medical insurance. We can see that the annual costs of those macromolecular drugs marketed in China are generally within RMB100,000, except ustekinumab. And to enter China’s national medical insurance, the annual cost shall be around RMB60,000, with successful cases including golimumab, tocilizumab, and Yisaipu, etc. As China’s national insurance becomes the biggest buyer of pharmaceuticals in China, Humira, Enbrel, and Actemra have been largely reduced prices to enter China’s national medical insurance and comply with the industry trend in China!
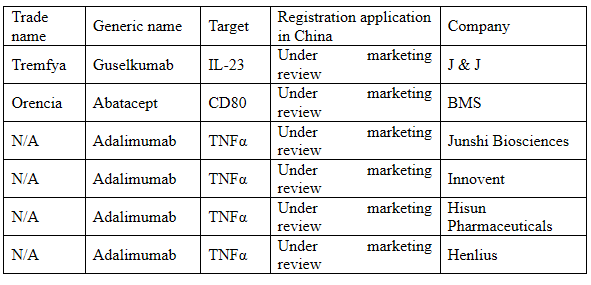
There are still 6 macromolecular drugs under marketing review at present, including the world’s first anti-IL-23 monoclonal antibody: guselkumab.
With the strong impacts from biosimilars and the initiative taken to reduce original drug prices, the Chinese autoimmune disease drug market is expected to undergo a reshuffle in the next 5 years. And:
1. The related enterprises will strive to enter China’s national medical insurance and medical insurance negotiations. With Yisaipu, Actemra, and Simponi as the benchmarks and yardsticks, those that enter China’s national medical insurance and medical insurance negotiation range will be those that win the Chinese market. Actemra (golimumab[SL1] ) is a very good example. Cosentyx (secukinumab) is also expected to be able to enter the negotiation range. And golimumab and tocilizumab will have promising market sales in the future.
2. The absolute market dominant position of Yisaipu will be changed and its market share will be severely eroded as a result of the strong impacts from Simponi, Cosentyx, Tremfya (not approved yet), and the newly approved adalimumab QLETLI.
3. The Chinese autoimmune disease drug market still has strong potential and will be gradually expanded as the diagnosis and treatment levels and medical insurance payment capacity improve.
-----------------------------------------------------------------------
Editor's Note:
To become a freelance writer of En-CPhI.CN,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@ubmsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


