Tim FreemanNovember 14, 2019
Tag: Freeman Technology , powder behaviour , Tim Freeman , Powder Flow , Flow Additives
Many powders have poor basic flow properties: They block in hoppers and die feed frames, exhibit inconsistent or pulsatile discharge rates, adhere to surfaces of equipment or don’t mix readily with other materials. In such cases, it is possible to reduce particle-particle friction and change the powder’s bulk resistance to movement by the addition of a lubricant powder. Magnesium Stearate (MgSt) is used extensively as a lubricant in the manufacture of pharmaceutical oral solid dosage forms. It is commonly added to formulations in low concentrations (typically < 1% w/w) immediately prior to tableting.
Over a range of processes, however, the relationship between flow additive and substrate is less well-understood. Due to the differing demands placed on powders by different processes, the relative influence of a given additive on a given substrate may not be consistent under all circumstances.
Preparation of Samples
Three different Magnesium Stearate (MgSt) formulations (Stear-O-Wet, Monohydrate & Dihydrate – Malinckrodt) were blended separately with five excipients (Avicel PH101 – FMC; Inhalac 230 & Granulac 70 – Meggle; C*Mannidex & C*Sorbidex – Cargill).
The blending was undertaken for a fixed period and rotational speed in a Turbula T2A mixer (Willy A Bachofen AG). The resultant blends were tested using an FT4 Powder Rheometer® to evaluate their Dynamic Flow, Bulk and Shear properties, and compared with the results generated for their respective substrates without lubricant.
The Relative Influence of Flow Additive Content on Flow Properties
Dynamic Testing: Basic Flowability Energy
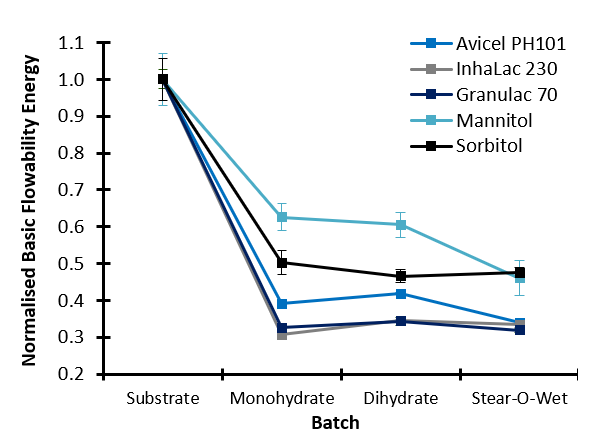
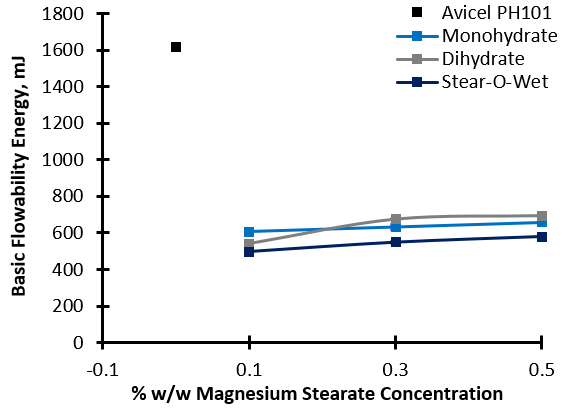
All lubricants significantly reduce the BFE of the substrates, indicating increased flowability in dynamic, forced flow operations such as feeding and blending, with the Inhalac 230 showing the greatest sensitivity to lubrication overall and Mannitol the least. Considering Avicel PH101 as the substrate, even small quantities of lubricant (0.1%) significantly improve the dynamic flow properties, but increases in concentration do not result in further enhancement.
Bulk Testing: Compressibility
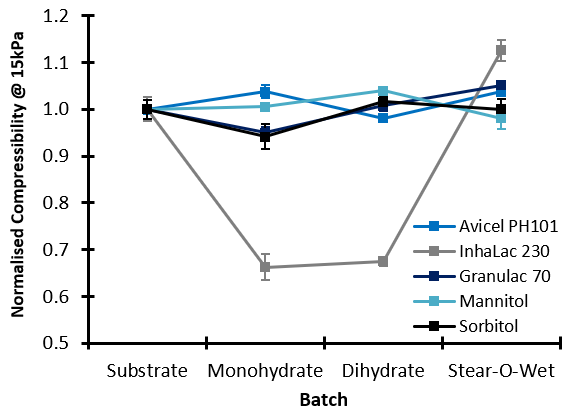
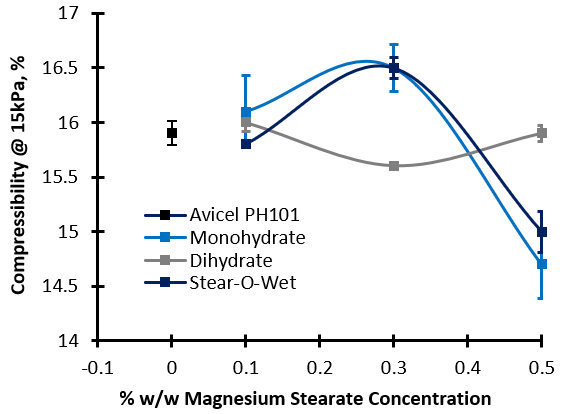
The addition of lubricant to Inhalac 230 caused significant changes in Compressibility. The Mono- and Dihydrates generated a reduction in Compressibility, but the addition of Stear-O-Wet caused an increase, indicating that the different lubricants interact with the substrate in different ways. Only moderate changes in Compressibility were observed for the other combinations of substrate and lubricant. Using Avicel PH101 as an example, the substrate showed a divergent response to the addition of different grades. Monohydrate and Stear-O-wet caused an increase in Compressibility at low concentration followed by a decrease as concentration increased. Dihydrate caused the opposite response. High Compressibility is often a sign of cohesivity within the bulk, and can prove detrimental to compaction operations such as tableting.
Shear Cell Testing
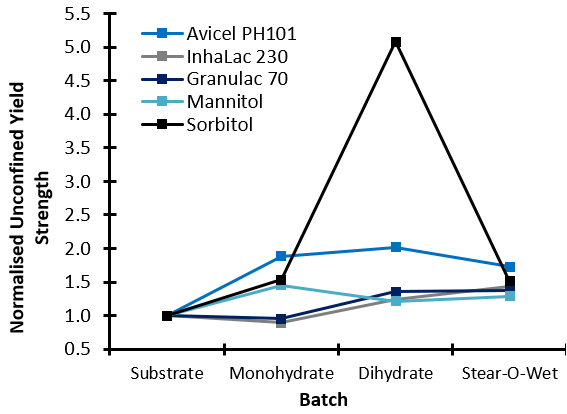
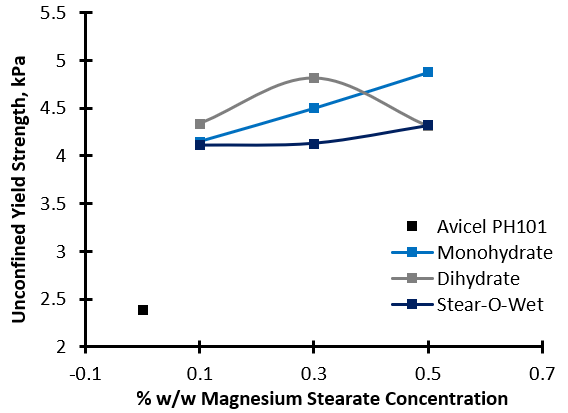
The Dihydrate and Stear-O-Wet typically increase the UYS of the substrates (drastically in the case of the Sorbitol/Dihydrate blend), indicating that these combinations would become more resistant to the onset of flow following storage (hence why lubricant is added towards the end of the process, and not in the hopper, where the effect may be detrimental). The influence of Monohydrate on UYS is dependent upon the substrate. Granulac 70 and Inhalac 230 both show a reduction in UYS, in contrast to the other three substrates. With Avicel PH101 as the substrate, the increase in UYS was shown to be not only dependant on the type of lubricant, but also on the concentration.
Conclusions
This study quantifies how the addition of a lubricant can dramatically change the flow properties of a range of typical pharmaceutical excipients, and how the concentration level and type of lubricant is key to optimising processability and cost-efficiency. It also demonstrates that lubricant/substrate interactions are complex, even for a simple two component system, and the influence of lubricants must be quantified with respect to process conditions.
Powder flowability is not an inherent material property, but is more about the ability of powder to flow in a desired manner in a specific piece of equipment. Successful processing demands that the powder and the process are well-matched and it is not uncommon for the same powder to perform well in one process but poorly in another. Rather than relying on single number characterisation to describe behaviour across all processes, the FT4’s multivariate approach simulates a range of unit operations, allowing for the direct investigation of a powder’s response to various process and environmental conditions.
Author Biography

Tim Freeman, Managing Director, Freeman Technology
Tim Freeman is Managing Director of powder characterisation company Freeman Technology for whom he has worked since the late 1990s. He was instrumental in the design and continuing development of the FT4 Powder Rheometer® and the Uniaxial Powder Tester. Through his work with various professional bodies, and involvement in industry initiatives, Tim is an established contributor to wider developments in powder processing.
Tim has a degree in Mechatronics from the University of Sussex in the UK. He is a mentor on a number of project groups for the Engineering Research Center for Structured Organic Particulate Systems in the US and a frequent contributor to industry conferences in the area of powder characterisation and processing. A past Chair of the American Association of Pharmaceutical Scientists (AAPS) Process Analytical Technology Focus Group Tim is a member of the Editorial Advisory Board of Pharmaceutical Technology and features on the Industry Expert Panel in European Pharmaceutical Review magazine. Tim is also a committee member of the Particle Technology Special Interest Group at the Institute of Chemical Engineers, Vice-Chair of the D18.24 sub-committee on the Characterisation and Handling of Powders and Bulk Solids at ASTM and a member of the United States Pharmacopeial (USP) General Chapters Physical Analysis Expert Committee (GC-PA EC).
www.micromeritics.com.cn
info@freemantech.com.cn
-----------------------------------------------------------------------
Editor's Note:
To become a freelance writer of En-CPhI.CN,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@ubmsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


