PharmaSources/1°CNovember 14, 2019
Tag: Opdivo , Keytruda , Tecentriq , Imfinzi , PD-(L)1
MSD released the Q3 financial report on Oct. 29, 2019. Its figures, especially the figures of its oncology business, were no longer shining, with keywords including Keytruda, Gardasil 9, and China.
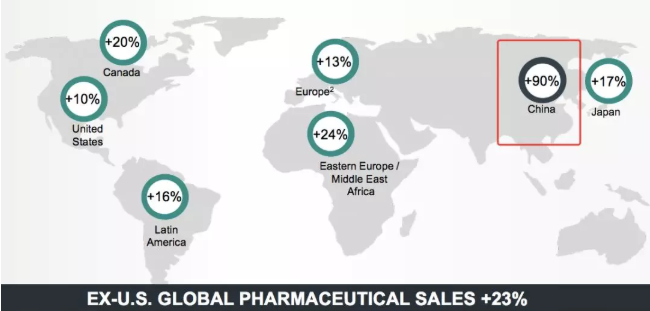
Source: MSD
MSD’s Keytruda is incomparable in its key indication: lung cancer. Its sales reached USD3.07 billion in 2019 Q3, growing by +62% year on year, and its sales reached USD7.973 billion in the first 9 months of 2019. According to my forecast, Keytruda’s sales will reach USD11 billion in 2019, to exceed Opdivo and Revlimid to rank the world’s second bestselling drug, and it will eventually become the new drug king by replacing Humira.
I’ve also noticed that the Chinese enterprise Jiangsu Hyamab Pharmaceutical Co., Ltd. applied for an anti-PD-L1 monoclonal antibody on Oct. 30, 2019 using IgG4kappa, S228P, ADCC weak and CDC weak, to join the 40+ Chinese applicants. And the patent of this anti-PD-L1 monoclonal antibody that can be queried is CN108699146A, PCTWO2017118321(A1), with the priority date being Jan. 4, 2016.
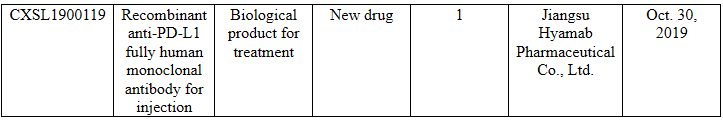
Source: CDE
Via this article, I’d like to review some important advances of anti-PD-(L)1 monoclonal antibodies in 2019 Q3, which involves Keytruda, Optivo, Tecentriq, and Imfinzi and also some noteworthy advances in China!
I. List of important advances in global anti-PD-(L)1 monoclonal antibodies:
Anti-PD-(L)1 monoclonal antibodies have revolutionized oncology! They largely extend patients’ 5-year survival rate, which is of milestone significance. And Keytruda has undoubtedly established strong market advantages, with increasingly rich real-world data, giving it unshakable advantages, while, Opdivo and Tecentriq follow Keytruda closely to become the other two products with strong competitiveness in this area!
Keytruda
1. MSD received positive EU CHMP opinion for the sBLA of Keytruda as first-line treatment for head and neck squamous cell carcinoma on Oct. 8, 2019;
2. Keytruda was approved as first-line treatment for non-small cell lung cancer (NSCLC) ([TPS]≥1%) in China on Oct. 2, 2019;
3. Keytruda’s KEYNOTE-522 clinical progress was updated on Sep. 29, 2019 about it as an adjuvant/neoadjuvant therapy for high-risk, early-stage TNBC patients;
4. On Sep. 17, 2019, the FDA approved Keytruda to be used in combination with LENVIMA (lenvatinib) to treat endometrial cancer.
5. Keytruda’s KEYNOTE-189, KEYNOTE-407 and KEYNOTE-021 subgroup analysis data were updated on Sep. 10, 2019;
6. Keytruda will be approved a new indication, i.e., CSCC, on June 29, 2020.
Opdivo:
1. CheckMate-9LA succeeded on Oct. 22, 2019, and CheckMate–227part1a and CheckMate-9LA are expected to support Opdivo to be approved as first-line therapy for NSCLC;
2. Opdivo’s ATTRACTION-3 was announced positive progress on Sep. 30, 2019: Opdivo could reduce patients’ death risk compared to chemotherapy;
3. Opdivo was approved as second-line therapy for head and neck squamous cell carcinoma in China on Sep. 30, 2019;
4. Opdivo was announced its 5-year overall survival data in treated NSCLC patients on Sep. 10, 2019, which was 13.4% compared to the 2.6% of docetaxel;
Tecentriq:
1. IMbrave150 succeeded on Oct. 21, 2019: Tecentriq+Avastin could bring significant overall survival and progression‐free survival benefits to patients with unresectable hepatocellular carcinoma compared to sorafenib;
2. Tecentriq’s IMvigor130 achieved positive progress as first-line treatment for bladder cancer on Sep. 30, 2019: Tecentriq could reduce patients’ disease progression risk in combination with chemotherapy;
3. On Sep. 27, 2019, Tecentriq’s IMpower110 achieved positive progress as first-line treatment for NSCLC: Tecentriq could bring overall survival benefits to (TC3/IC3-WT) NSCLC patients compared to chemotherapy!!!!
4. Tecentriq was approved as first-line treatment for NSCLC in the EU upon IMpower130 on Sep. 6, 2019;
Imfinzi:
1. AstraZeneca updated its high-profile POSEIDON on Oct. 28, 2019: IMFINZI+tremelimumab in combination with chemotherapy could improve patients’ PFS to reach a primary endpoint of the clinical trial compared to chemotherapy;
2. Imfinzi’s NEPTUNE was updated on Aug. 21, 2019, which failed;
II. Back to China: 40+ IND applications for the anti-PD-L(1) monoclonal antibodies
As mentioned above, Hyamab Pharmaceutical’s fully human anti-PD-L1 monoclonal antibody is among the 40+ anti-PD-L(1) applied for in China, with main characteristics as follows:
1. Discovery method: fully human monoclonal antibody screened on genetically engineered mice through the hybridoma technique;
2. Hyamab Pharmaceutical uses IgG4kappa, S228P, ADCC weak and CDC weak, unlike the marketed anti-PD-L1 monoclonal antibodies;
3. The company prompts the attention to cloning 78A11F6 and 73D3G2 and seeing the patent for the sequence details.
Besides the IND applications for 40+ anti-PD-L(1) monoclonal antibodies in China, it’s also worth noting that there have been 5 anti-PD-L(1) monoclonal antibodies approved for marketing in China and all have been commercialized, with rapid sales growth far quicker than other innovative drugs marketed in China.
The first anti-PD-L1 monoclonal antibody will be launched very soon in China as 2019 is approaching the end. The number of anti-PD-L(1) monoclonal antibodies approved for marketing in China is expected to reach 8 by the end of 2019.
PS: I’ve found that the number "3" has strong magic. Chapter 42 of the Tao Te Ching has a classic sentence, "The Tao begot one. One begot two. Two begot three. And three begot the ten thousand things." 3 is a number worth delving into. It is used in the consistency evaluation and "4+7", etc. in China. And for anti-PD-(L)1 monoclonal antibodies, will the number of approved PD-(L)1 indications be linked with 3?
About the author: 1°C, a practitioner in the pharmaceutical industry, who wants to write professional articles that are increasingly heartwarming, serve more people through medical knowledge and break information knowledge barriers!
-----------------------------------------------------------------------
Editor's Note:
To become a freelance writer of En-CPhI.CN,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@ubmsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


