En-CPhI.CNNovember 06, 2019
Tag: API , C2 Pharma , C2P , Digoxin
C2 PHARMA s.a.r.l. (C2P), a Luxembourg-based phytochemical and chemical pharmaceutical manufacturing and distribution group, continues to build its active pharmaceutical ingredient (API) and botanical extracts product portfolio. The most recent addition enhances the previously announced Digoxin API product offer with the inclusion of a micronized grade for Digoxin from two different manufacturing sites. Multiple niche APIs and botanical extract products are also currently in technical transfer or under development, including Cannabidiol (CBD) and Tetrahydrocannabinol (THC).
C2P has followed through on plans to file for the US drug master file (US-DMF) of Digoxin (Micron) manufactured at its partner and contract manufacturing organization (CMO) site Laurus labs in India at the end of October. Plans are further confirmed for filing the CEP (Certificate of Suitability to the monographs of the European Pharmacopoeia) by the end of November 2019. For the Digoxin (Micron 10), manufactured at CMO site Nobilus in Poland, the validation campaign has been successfully completed and the US-DMF and the CEP fillings are planned for the first half of 2020. Samples for micronized Digoxin from both sites are currently available.
"The micronized grade of digoxin API has been developed to meet stringent specifications for particle size distribution less than 10 μm. These two APIs will integrate C2P’s existing portfolio of four different grades of Digoxin API, including our injectable one." said Andrew Badrot, C2P’s CEO. "This extension is part of our long-term plan to act as responsible stewards of the Digoxin API and secure the supply chain for this complex and essential API by offering our customers a redundant and fully independent supply chain via two CMO manufacturing sites, one at Nobilus in Poland and one at Laurus Labs in India."
As the team works to continuously expand the API product portfolio, C2P has confirmed the successful validation campaign of Homatropine Hydrobromide. Samples are available and regulatory fillings will follow in mid-2020.
C2P has also revamped its R&D product pipeline and is actively working on six tech transfers, an additional five APIs in various phases of development and 19 APIs are in early feasibility.
"In addition to the Digoxin and Homatropine expansion, we have a full portfolio of API and botanical extracts that are coming to market with our high-quality virtual manufacturing approach, which is currently unmatched in the industry. Our CBD franchise is progressing, and we are ramping up for commercial production of CBD as an API," added Andrew Badrot.
C2P’s current R&D pipeline follows:
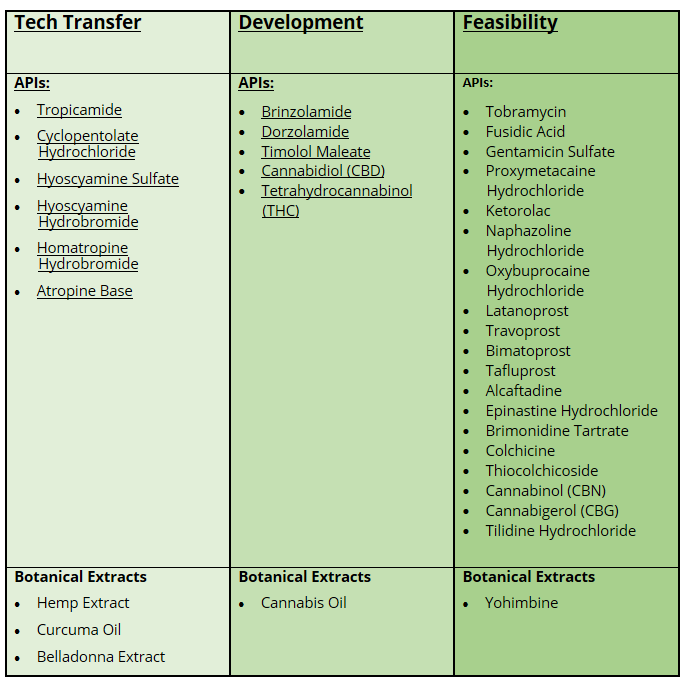
A full list of products currently available can be seen on the website at: https://www.c2pharma.com/apis/.
About C2 PHARMA
Established in 2014, C2 PHARMA is a Luxembourg-based manufacturer and distributor of active pharmaceutical ingredients (APls) from phytochemical and chemical origins. The company focuses on developing APIs that are niche, complex, difficult to manufacture, challenging to sell or to transport by combining best-in-class manufacturing, quality oversight and technical expertise. The current API product portfolio includes atropine, digoxin, homatropine, pilocarpine, hyoscine butylbromide and more than 20 APIs are at various stages of R&D development. Customized GDP cold-chain logistics solutions are offered through a specialized affiliate: Logistics4Pharma. R&D-scale contract synthesis services, analytical services, phytochemical profiling and impurities manufacturing are provided via its affiliate ASM Research Chemicals.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


