PharmaSources/CaicaiNovember 05, 2019
Tag: biosimilar , adalimumab , Bio-Thera
Edtor's Notes:
On November. 7th, Bio-Thera Solutions, Ltd., a clinical-stage pharmaceutical company, Onannounced that the China National Medical Products Administration (NMPA) has approved QLETLI® (格乐立®) for all eligible indications of the reference product, Humira® (adalimumab), in China.
Below are a previous article:
The marketing application of Bio-Thera’s adalimumab (acceptance No.: CXSS1800018) has recently been under the "under approval" status, which is expected to be approved for marketing soon for treating ankylosing spondylitis (AS). Bio-Thera has aroused discussions owing to its application for listing on the Sci-tech Innovation Board of China when it does not generate any revenue. If the company can successfully produce the first adalimumab biosimilar, this will be great news before its listing.
Annual sales of USD20.5 billion and 3 indications in China of the global drug king
TNF-ɑ (tumor necrosis factor-ɑ) is a known proinflammatory cytokine that mainly regulates immune cells. TNF-ɑ receptors can transduce survival and death signals to cells and play an important role in immune response regulation.
The original drug adalimumab (trade name: Humira) is a fully human anti-TNF-ɑ antibody of AbbVie, which was approved for marketing in the U.S. in 2002. It has been successively approved for 10 indications for more than a decade, including rheumatoid arthritis (RA), AS, plaque psoriasis, Crohn's disease (CD), juvenile idiopathic arthritis, and uveitis, etc. Its annual sales have been ranking first in the world for 7 consecutive years, with the global sales revenue of USD20.5 billion in 2018. And its therapeutic effects in autoimmune diseases have been extensively demonstrated.
The original drug adalimumab was approved for marketing in China in 2010 and is now approved 3 indications, i.e., RA, AS, and plaque psoriasis; and its CD indication has been completed the Phase III clinical trial. The original drug adalimumab was included in the List of the Second Batch of Overseas New Drugs Catering to Clinical Urgent Needs by the CDE in May 2019 for treating uveitis. This indication may be submitted data according to the Work Procedures for the Review and Approval of Overseas New Drugs Catering to Clinical Urgent Needs and directly filed the marketing application and will enjoy the priority review and approval.
The median bid-winning price of this original drug was RMB7,586/piece (40mg/0.8ml) in China in 2018. The cost of a patient using the drug according to the package insert will be nearly RMB200,000 a year. Such a high cost has restricted the accessibility of the drug to the broad patients. This original drug has not entered China’s national medical insurance drug catalog and only entered the medical insurance drug catalogs for major and serious diseases in some places, such as Chengdu City Medical Insurance Drug Catalog for Major and Serious Diseases, Shenzhen City Supplementary Medical Insurance Drug Catalog for Major and Serious Diseases, and Qingdao City Catalog of Special Drugs, Special Medical Consumables and Precision Diagnosis and Treatment Items of Supplementary Medical Insurance.
Size of the adalimumab biosimilar market may reach RMB11.5 billion in China in 2030
According to a report by Frost & Sullivan, China’s adalimumab biosimilar market is expected to increase to RMB4.7 billion in 2023 and reach RMB11.5 billion in 2030.
Size of China’s Adalimumab Biosimilar Market in 2020-2030
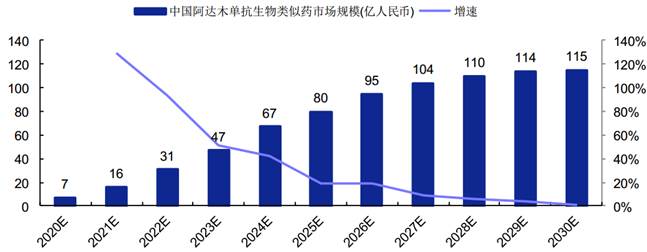
中国阿达木单抗生物类似药市场规模(亿人民币) 增速 | Size of China’s adalimumab biosimilar market (RMB one hundred million) Growth |
(Source: Bio-Thera’s prospectus, Frost & Sullivan)
Bio-Thera, Hisun Pharmaceutical, Innovent, and Henlius have filed the relevant marketing applications
The antibody sequence patent of the original drug adalimumab expired in China in 2017. It’s difficult for the families of many autoimmune disease patients who require long-term medication to afford the expensive original drug price. According to the CDE’s data, 15 Chinese companies have conducted adalimumab biosimilar clinical trials by May 2019.
Wherein, Bio-Thera was the first in filing the marketing application and its product was included in the priority review in Nov. 2018.
Hisun Pharmaceutical, Innovent, and Henlius have successively filed the marketing applications following Bio-Thera. Adalimumab biosimilars of Junshi Biosciences, Tonghua Dongbao, and Sinocelltech have entered Phase III clinical trials.
Adalimumab Biosimilars that have been Filed NDAs or Entered Phase III Clinical Trials in China
Product name | Company name | Status | Indication | CDE acceptance date/first publication date of clinical trial |
BAT1406 | Bio-Thera | NDA | AS | Aug. 27, 2018 |
HS016 | Hisun Pharmaceutical | NDA | AS | Sep. 25, 2018 |
IBI303 | Innovent | NDA | AS | Nov. 15, 2018 |
HLX03 | Henlius | NDA | Plaque psoriasis | Jan. 28, 2019 |
Phase I clinical trial | RA | Dec. 9, 2016 | ||
UBP1211 | Junshi Biosciences | Phase III clinical trial | RA | May 27, 2017 |
DB101 | Tonghua Dongbao | Phase III clinical trial | Plaque psoriasis | Feb. 26, 2019 |
Phase I clinical trial | RA | Aug. 18, 2017 | ||
SCT630 | Sinocelltech | Phase III clinical trial | Plaque psoriasis | June 6, 2019 |
(Source: Bio-Thera’s prospectus)
Number of Chinese patients with different indications of adalimumab
RA:
RA is an autoimmune disease with the main clinical manifestations of chronic, progressive, multiple, invasive joint synovitis and extra-articular lesions. The number of RA patients in China grows steadily. According to the report by Frost & Sullivan, the said number grew from 5.745 million to 5.878 million from 2014 to 2018, with the compound annual growth rate (CAGR) of 0.6%. RA patients will continue to increase due to the continuous deterioration of risk factors such as aging and environment. Adalimumab is an effective second-line therapy for RA.
AS:
AS is a chronic inflammation that occurs at the spine position of patients, with a long course. Patients will lose most of their ability to move after the disease deteriorates. Susceptibility gene and family history are the main pathogenic factors of AS, therefore, the number of Chinese AS patients is relatively stable.
According to the report by Frost & Sullivan, the number of Chinese AS patients increased from 3.76 million to 3.85 million from 2014 to 2018, with the CAGR of 0.6%. This number is expected to increase to 3.955 million in 2023, with the CAGR of 0.5%. This number will subsequently grow at a CAGR of 0.4% to reach 4.054 million in 2030.
Number of Chinese AS Patients in 2014-2030
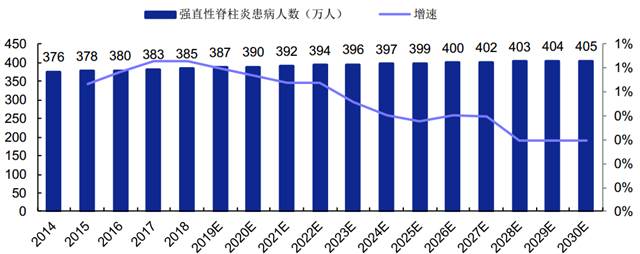
强直性脊柱炎患病人数(万人) 增速 | Number of Chinese AS Patients Growth |
(Source: Bio-Thera’s prospectus, Frost & Sullivan)
Psoriasis:
Psoriasis is an autoimmune skin disease mainly mediated by T cells as a result of the body's immune system being stimulated by multiple pathogenic factors under the background of polygenic inheritance, wherein, plaque psoriasis is the most common type of psoriasis.
According to the report by Frost & Sullivan, the number of Chinese plaque psoriasis patients increased from 5.786 million to 5.902 million from 2014 to 2018, with the CAGR of 0.5%. This number will grow as the population grows and will separately reach 6.062 million and 6.168 million in 2023 and 2030.
Number of Chinese Plaque Psoriasis Patients in 2014-2030
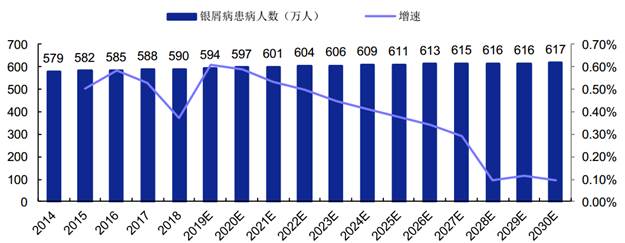
银屑病患病人数(万人)
增速 | Number of plaque psoriasis patients (ten thousand) Growth |
(Source: Bio-Thera’s prospectus, Frost & Sullivan)
CD:
CD is a gastrointestinal chronic inflammatory granulomatous disease with the cause unknown and has clinical characteristics of abdominal pain, diarrhea, loss of weight, abdominal mass, sinus tract formation, and ileus, with a chronic course and repeated attacks. According to the report by Frost & Sullivan, the CD incidence has been gradually increasing in China in recent years. The number of Chinese patients grew from 68,000 to 120,000 from 2014 to 2018, with the CAGR of 15.2%, and this number will separately reach 188,000 and 283,000 in 2023 and 2030.
Number of Chinese CD Patients in 2014-2030
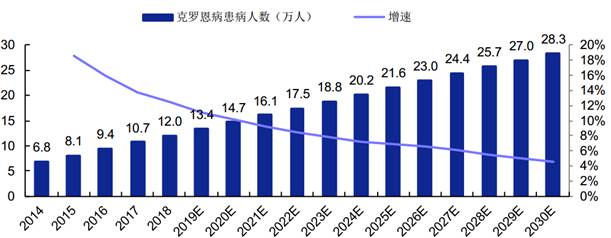
克罗恩病患病人数(万人) 增速 | Number of CD patients (ten thousand) Growth |
(Source: Bio-Thera’s prospectus, Frost & Sullivan)
The Clinical Trial Design Considerations for Adalimumab Injection Biosimilars issued by the CDE on Mar. 1, 2019 mentions, "If a certain crowd with the approved indication of the original drug has been completed the biosimilar systematic comparability study, the candidate drug may seek the approval for the original drug’s other approved indications with the same mechanism of action based on the existing data and information." Therefore, after Bio-Thera’s adalimumab is approved for marketing in China, the company may further extend the indications to all indications of the original drug approved in China.
Bio-Thera is the second pharmaceutical company to be listed on the Sci-tech Innovation Board by choosing the standard (5)
According to Bio-Thera’s prospectus, Bio-Thera meets the listing standard stipulated in (5), Paragraph 2, Article 22 of the Rules of the Shanghai Stock Exchange for Examination of the Issuance and Listing of Stocks on the Sci-tech Innovation Board: With the estimated market capitalization of not less than RMB4 billion, the main business or product required to be approved by the relevant department of the state, big market space and staged achievements made. Pharmaceutical industry enterprises are required to have at least one core product approved for Phase II clinical trials, and other enterprises that meet the Sci-tech Innovation Board positioning are required to possess clear technical advantages and meet the corresponding conditions.
According to relevant data, Bio-Thera is the second pharmaceutical company to be listed on the Sci-tech Innovation Board by choosing the standard (5) following Zelgen c and also the second pharmaceutical company to be listed on the Sci-tech Innovation Board, which is still at a loss.
Related News:
Humira Topped for Seven Times in Sales and Remaining Global Drug King
Source: Bio-Thera’s prospectus
Caicai, a Master of Pharmacy from Shanghai Jiaotong University, used to work in the Institute of Science and Technical Information. Currently as a practitioner in the drug surveillance system, she is good at interpreting industry regulations, pharmaceutical research developments, etc.
-----------------------------------------------------------------------
Editor's Note:
To become a freelance writer of En-CPhI.CN,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@ubmsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


