PharmaSources/zhulikou431November 14, 2019
Tag: Injection , injection consistency evaluation
Technicians of pharmaceutical enterprises have significantly more to learn in the second half of 2019 with the promulgation of the Drug Administration Law (2019 Revision). The National Medical Products Administration of China (NMPA) issued the drafts for comment of three important laws before China’s National Day, separately, the Provisions for Drug Registration, Measures for the Supervision over and Administration of Pharmaceutical Production, and Measures for the Supervision over and Administration of Pharmaceutical Product Operation. The NMPA also issued on Oct. 15 the NMPA Department of Comprehensive Affairs, Planning, and Finance Affairs Soliciting Public Opinions on the Technical Requirements for the Quality and Efficacy Consistency Evaluation of the Generic Drugs of Chemical Drug Injections (Draft for Comment) and Requirements for the Application Data of the Quality and Efficacy Consistency Evaluation of the Marketed Generic Drugs of Chemical Drug Injections (Draft for Comment).
Regarding the injection consistency evaluation regulation, the Center for Drug Evaluation, NMPA (CDE) has issued the Notice on Soliciting Public Opinions on the Technical Requirements for the Consistency Evaluation of the Marketed Chemical Generic Drugs (Injections) early on Dec. 22, 2017. This draft for comment has not been further revised and improved within nearly 2 years, however, some excellent enterprises in the pharmaceutical industry of China have already started the injection product consistency evaluation work, such as Shandong Qilu Pharmaceutical, and Sichuan Huiyu Pharmaceutical.
See the following table for the incomplete statistics of the relevant pharmaceutical products that have passed the consistency evaluation by the end of Dec. 2018:
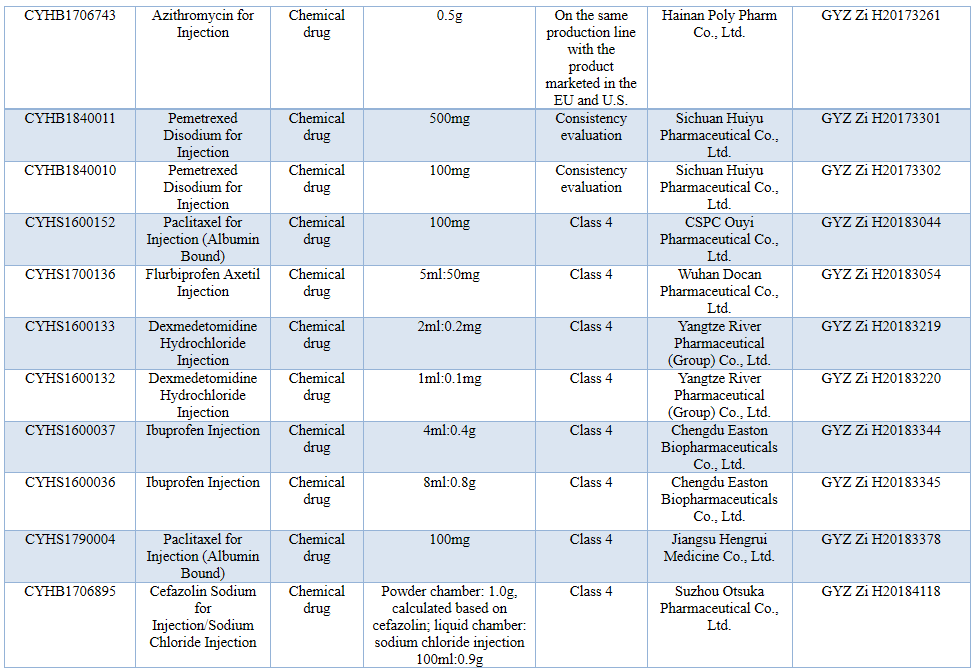
Moreover, the NMPA has continued to increase the varieties of aseptic preparations (including eye drops and injections) in the documents of lists of reference listed drugs (RLDs) of chemical generic drugs issued in succession, for example, the 24th list of RLDs of chemical generic drugs issued by the NMPA on Sep. 16, 2019 includes the RLDs of many aseptic preparations and many aseptic preparation varieties such as bimatoprost and timolol eye drops, prednisolone acetate eye drops, and phloroglucinol injection.
Based on the above policy signals and in combination with the latest documents on injection consistency evaluation issued today by the NMPA, it can be predicted that the injection consistency evaluation will be comprehensively implemented in 2019 or at the beginning of 2020.
I’d like to analyze the points and give my suggestions in combination with the Technical Requirements for the Quality and Efficacy Consistency Evaluation of the Generic Drugs of Chemical Drug Injections (Draft for Comment) and Requirements for the Application Data of the Quality and Efficacy Consistency Evaluation of the Marketed Generic Drugs of Chemical Drug Injections (Draft for Comment) issued today, in the hope of helping the industry practitioners cope with it.
Read more:
Zhulikou431, as a senior engineer, PDA member, ISPE member, ECA member, PQRI member, senior aseptic GMP expert, has deep knowledge in aseptic process development and verification, drug development and registration, CTD document writing and review, regulatory audit, international certification, international registration , quality system construction and maintenance, as well as sterile inspection, environmental monitoring and other fields. In recent years, he has focused on the analysis of trends in the macro pharmaceutical field and the risk management of pharmaceutical enterprise mergers and acquisitions projects.
-----------------------------------------------------------------------
Editor's Note:
To become a freelance writer of En-CPhI.CN,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@ubmsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


