PharmaSources/DopineOctober 29, 2019
Tag: Injection , Salubris , Teriparatide , osteoporosis drug market
The marketing application of Salubris’ Teriparatide for Injection has been finally approved recently after 2 rounds of data supplementation and is now under the status of "to be issued certificate", which means that the second teriparatide biosimilar will arrive in China and is expected to making waves in the market.
As a human parathyroid hormone (PTH) analog, teriparatide was first developed by Eli Lilly and has the same structure with the 34 N-terminal amino acid sequence of PTH. According to research, teriparatide can stimulate bone formation, improve bone density and quality and reduce patients’ risks of vertebral and non-vertebral fractures and is approved by the FDA to treat osteoporosis in postmenopausal women at highest risk for fracture and men at high fracture risk. Marketed in the U.S. in 2002, it was approved in China in 2011. Teriparatide is the only osteoporosis drug approved by the FDA, which can promote bone formation. Currently, the bone formation promoting drugs in China include Eli Lilly’s teriparatide (trade name: Forsteo) and also Shanghai United Cell Biotechnology’s teriparatide biosimilar (approved in Mar. 2017, with the trade name: OstioGen).
As a blockbuster drug in the osteoporosis drug market, the global sales of Eli Lilly’s Forsteo had been increasing by 2017 since marketed; the global sales have exceeded USD1 billion since 2012 and peaked in 2017 to reach USD1.749 billion. See the following chart for the details. However, the sales of Forsteo are barely satisfactory in China mainly because of the expensive price and excessive treatment cost (the latest bid-winning price queried on Insight Database is RMB5,338.50, with the whole treatment course 24 months costing RMB128,000, which is not covered by China’s national medical insurance), which is only secondary prevention against fracture risk increase or for serious osteoporosis patients not responding to first-line drugs.
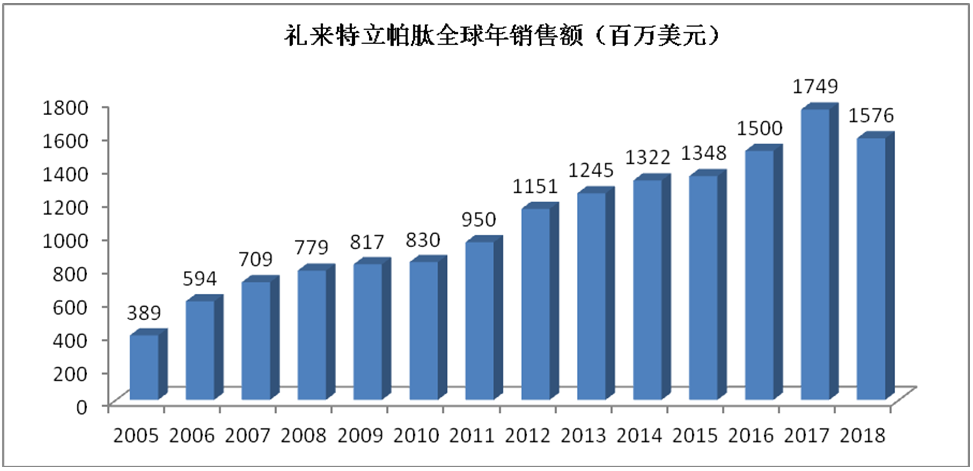
Annual Global Sales of Eli Lilly’s Teriparatide (USD One Million)
Sales (USD One Million)
The annual sales of Forsteo have not made a large-scale breakthrough, however, it still dominates the bone formation promoting drug market in China. China is the country with the largest number of osteoporosis patients, which is currently about 90 million; the market space of teriparatide calculated according to 10,000 people receiving treatment every year in China is expected to reach RMB 500 million to RMB 1 billion. The sales of teriparatide in China in recent years are as shown in the following chart. The sales grew gradually, however, the market space of teriparatide is far from reaching the expectation as seen from the chart. And the market of teriparatide has bigger room for expansion with the aging of population and consumption upgrade in China.
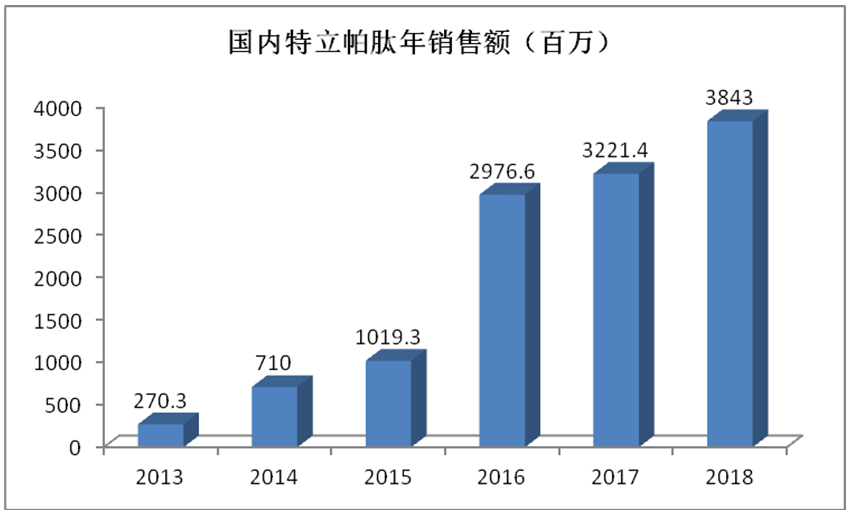
Annual Sales of Teriparatide in China (RMB One Million)
Sales (RMB One Million)
Teriparatide products marketed in China so far only include Forsteo and OstioGen. With the marketing of Salubris’ Teriparatide for Injection, the Chinese-produced teriparatide products are expected to increase their market shares upon price advantage, however, which Chinese-produced teriparatide is better depends on their respective price and efficacy. In addition to the above two teriparatide biosimilars, many enterprises are laying out the market, wherein, GeneTech Pharm has filed the marketing application, and Dongguan Polygene Biotech has completed the relevant Phase III clinical trial. The bone formation promoting drug market in China is expected to see a situation of quadripartite confrontation very soon, which will churn the osteoporosis drug market.
About osteoporosis and the drugs
Osteoporosis is a systemic bone disease characterized by low bone mass and microarchitectural deterioration of bone tissue, leading to enhanced bone fragility. It can be divided into primary and secondary osteoporosis, wherein, the primary osteoporosis can be further divided into type I (postmenopausal osteoporosis), type II (senile osteoporosis), and idiopathic type (including juvenile type). The number of osteoporosis patients is huge in China, and the size of the osteoporosis drug market reached up to RMB26 billion in 2016.
Osteoporosis drugs mainly include calcium absorption promoting drugs, bone resorption (or loss) inhibiting drugs, and bone formation promoting drugs. Wherein, calcium absorption promoting drugs include calcium carbonate D3, and calcium acetate, etc., mainly used in children and teenagers, which are easily accessible but do not have significant therapeutic effects, with the overall size reaching over RMB10 billion, accounting for a large share in the osteoporosis drug market; bone resorption (or loss) inhibiting drugs are represented by calcitriol, zoledronic acid, and salmon calcitonin, which have good effects of promoting calcium absorption, however, their annual sales are not as good as calcium absorption promoting drugs, with the annual sales of calcitriol and zoledronic acid of about RMB3 billion and those of salmon calcitonin of about RMB1 billion; bone formation promoting drugs include teriparatide and its analogs, which mainly treat serious osteoporosis patients insensitive to other therapeutic drugs and the increasingly younger postmenopausal female patients with osteoporosis and are expected to cure osteoporosis, however, the treatment costs are high, and their future market space is huge.
-----------------------------------------------------------------------
Editor's Note:
To become a freelance writer of En-CPhI.CN,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@ubmsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


