En-CPhIOctober 17, 2019
Tag: DPI , Tim Freeman , Powder Rheology
Dry Powder Inhalers (DPIs) are used to deliver a controlled dose of active pharmaceutical ingredient (API) to the deep lung. An excipient transfers the fine particles of the API from the capsule before being trapped in the throat and subsequently swallowed as the API continues to the lungs.
DPI performance will be dependent on several powder properties, including the response to air, permeability, inter-particular mechanical locking and friction. By measuring the rheological properties of DPI formulations, the properties of generic products can be matched to their branded counterparts.
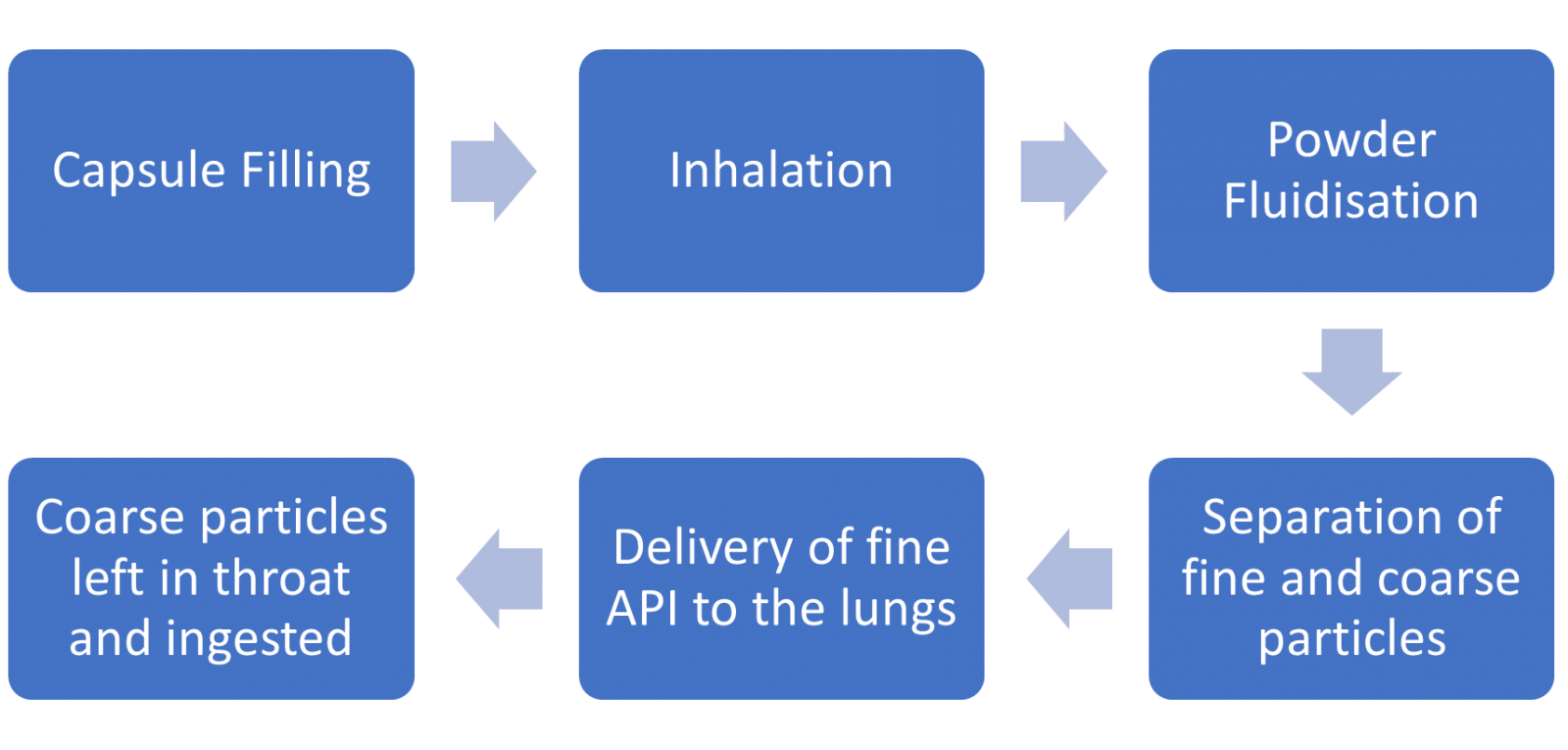
It is also important that the capsule filling method, and the inhaler geometry and mechanism are similar as any changes to the production or operation techniques could result in variation in performance and efficacy.
Process Relevant Powder Characterisation
For DPIs, the quantity of powder that reaches the deep lung, the delivered dose, must be controlled. For effective transport of the API into the deep lung it is necessary that the powder disperses in the inhaled air stream. Aeration properties of the formulation, such as the powder’s response to the introduction to air, the ability of the powder to fluidise and the cohesive forces between powder particles, are therefore vital.
In order to determine the Fine Particle Dose (FPD), i.e. mass of particles smaller than 5µm, it is typically necessary to conduct a range of extensive laboratory testing. However, in previous studies, FPD has been shown to exhibit a strong correlation with properties such as Aerated Energy (AE), Compressibility and Permeability [1,2]. AE is shown in Figure 1 shows an example of this for AE.
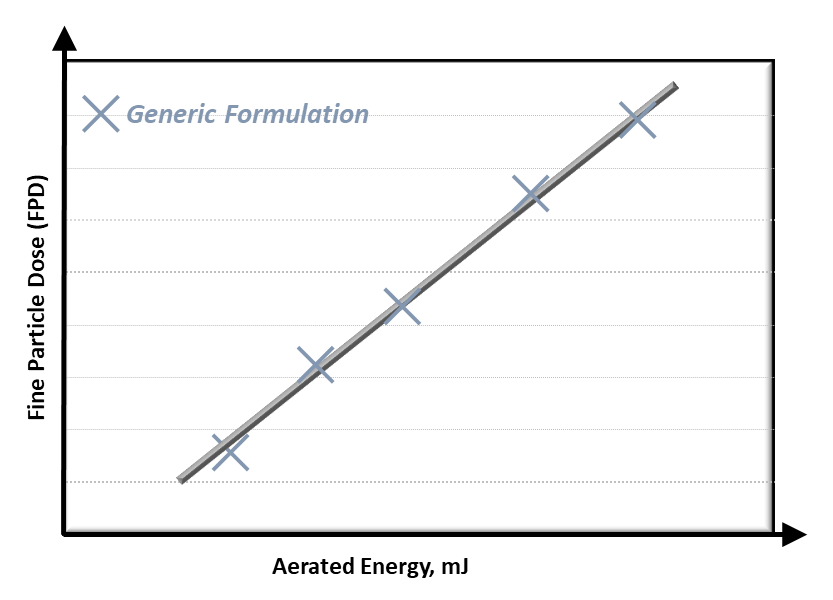
Figure 1: Fine Particle Dose (FPD) correlates with Aerated Energy.
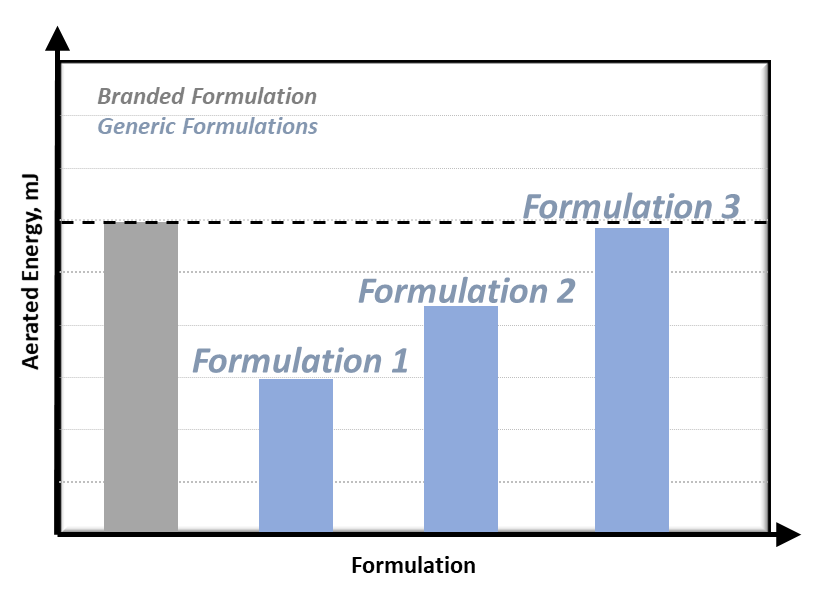
Figure 2: Matching Generic Formulation properties to Branded Formulation.
Therefore, when a new Generic Formulation is produced, the AE can be compared to that of the Branded Formulation, as shown in Figure 2. By adjusting the formulation as necessary, for example increasing fines content, the AE of the Generic Formulation can be matched to the Branded Formulation to ensure similar performance in the application.
By matching generic DPI formulations to branded products, manufacturers can be more confident of producing formulations which will have properties that are conducive to comparable performance, e.g. uniform dosage and efficient transport of API to the lungs.
References
[1] Kinnunen et al., AAPS PharmSciTec, 2014.
[2] Pitchayajittipong et al., Int. J. Pharm., 2010.
Author Biography

Tim Freeman, Managing Director, Freeman Technology
Tim Freeman is Managing Director of powder characterisation company Freeman Technology for whom he has worked since the late 1990s. He was instrumental in the design and continuing development of the FT4 Powder Rheometer® and the Uniaxial Powder Tester. Through his work with various professional bodies, and involvement in industry initiatives, Tim is an established contributor to wider developments in powder processing.
Tim has a degree in Mechatronics from the University of Sussex in the UK. He is a mentor on a number of project groups for the Engineering Research Center for Structured Organic Particulate Systems in the US and a frequent contributor to industry conferences in the area of powder characterisation and processing. A past Chair of the American Association of Pharmaceutical Scientists (AAPS) Process Analytical Technology Focus Group Tim is a member of the Editorial Advisory Board of Pharmaceutical Technology and features on the Industry Expert Panel in European Pharmaceutical Review magazine. Tim is also a committee member of the Particle Technology Special Interest Group at the Institute of Chemical Engineers, Vice-Chair of the D18.24 sub-committee on the Characterisation and Handling of Powders and Bulk Solids at ASTM and a member of the United States Pharmacopeial (USP) General Chapters Physical Analysis Expert Committee (GC-PA EC).
www.micromeritics.com.cn
info@freemantech.com.cn
-----------------------------------------------------------------------
Editor's Note:
To become a freelance writer of En-CPhI.CN,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@ubmsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


