PharmaSources/Richard ChaiSeptember 06, 2019
Tag: cleaning , Richard Chai , Biofilm Lifecycle , STERIS , Remediation Procedures
Biofilm formation in process equipment presents challenges and may lead to serious consequences in GMP related manufacturing. The presence of a biofilm, which may consist of Gram-positive, Gram-negative bacteria, mold, and bacterial spores on process equipment can affect the safety, identity, strength, quality, or purity of products manufactured with this equipment. Such effects can lead to adulterated product which may pose risks to consumers, and lead to regulatory action.

Understanding Biofilm Lifecycle and Remediation Procedures
The FDA regulation on microbial contamination is:
"21 CFR 211.113 Control of Microbiological Contamination"
(a) Appropriate written procedures, designed to prevent objectionable microorganisms in drug products not required to be sterile, shall be established and followed.
(b) Appropriate written procedures, designed to prevent microbiological contamination of drug products purporting to be sterile, shall be established and followed. Such procedures shall include validation of all aseptic and sterilization processes."
Microbial contamination of products can often lead to product recall. An example of recent product recall due to microbial contamination is shown below.
"The company states that a "small percentage" of its products that were manufactured between August 1, 2017 and April 2018 tested positive for microbial contamination. In July 2018, the company announced it was recalling four lots of Aquaflora Candida HP9, Lymph Detox, and Baby Teething liquids because Pseudomonas brenneri, Pseudomonas fluorescens, and Burkholderia multivorans were found during a routine FDA inspection."(1)
The FDA regulation and product recall example cited above demonstrate that microbial contamination is clearly a concern with pharma packaging machinery manufacturers and regulators. It is expected that any microbial contamination issues on site need to be dealt with promptly, with thorough investigations and implementation of robust corrective and preventive actions.
This article looks at the lifecycle of biofilm, conditions or factors that can lead to the formation of biofilm, and the methodology for biofilm remediation. Understanding these factors enables manufacturers to pro-actively ensure suitable procedures are in place to minimize the risk of biofilm.
Biofilm Lifecycle
Microorganisms have primarily been characterized as planktonic, i.e., unattached or freely suspended single cells, and characterized based on their metabolic and physical growth characteristics in culture media. More recently it has been shown that microorganisms are seldom found in natural settings in this planktonic state, rather they can and do attach to and grow on exposed surfaces in complex microbial communities, which led to studies revealing the nature of surface-associated microorganisms (biofilms).
What exactly is biofilm? Biofilm is generally composed of multiple micro-organisms encased in slimy complex matrix of polysaccharides, nucleic acids and proteins known as extracellular polymeric substances (EPS).
There are three main stages in the biofilm lifecycle (refer to diagram 1: Biofilm Lifecycle below).
Diagram 1: Biofilm Lifecycle
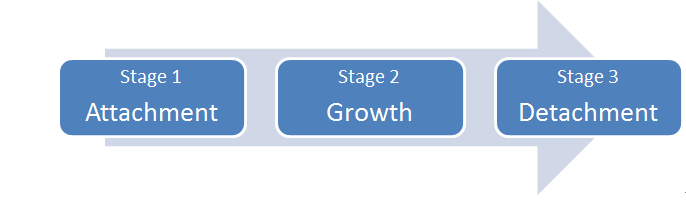
Stage 1: Attachment
Free-floating, or planktonic, bacterial cells can attach to surfaces in moist environments and start to excrete the slimy, glue-like substance known as EPS. The microorganisms attached to the surface can be a single bacterial species, or more commonly are comprised of multiple species of bacteria, as well as fungi, algae, yeasts, protozoa or other microorganisms, along with cellular debris and corrosion products.
Stage 2: Growth
Excretion of EPS allows biofilm community to develop quickly into a complex, three-dimensional (3D) structure that can be as thin as a few cell layers or a few inches thick, depending on environmental conditions.
Stage 3: Detachment
Small or large clumps of cells and debris can detach from the 3D structure, allowing the bacteria to spread and attach to another surface or another biofilm community downstream from the original community.
Biofilm contamination usually presents itself as a persistent contamination that can be detected following remediation attempts. Contamination may appear under control for a time, however, it can be detected again due to ineffective removal/remediation as some of the biofilm sloughs off or detaches.
Contributing Factors to Biofilm Formation
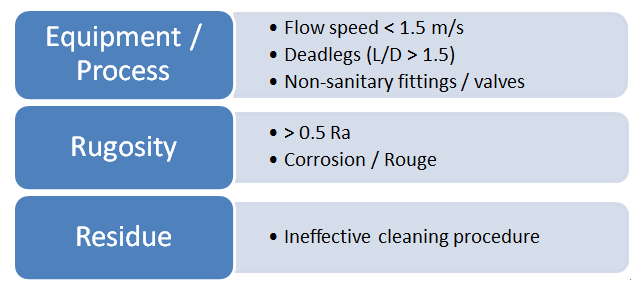
Diagram 2 shows the factors that could lead to the formation of the biofilm in process equipment.
• Equipment / Process: The flow velocity in the transfer pipes of less than 1.5m/s can increase the probability of microorganisms attaching to the surface of the pipes. Dead legs are sites in process equipment where the liquid can become stagnant and there is no proper exchange of cleaning fluids and water. The presence of dead legs with the ratio of length to diameter (L/D) more than 1.5 is not desirable as this will facilitate the formation of stagnant liquid or trapping of residues or microorganisms. Non-sanitary valves tend to trap residues and reduce cleanability. Sanitary valves such as diaphragm valves or pinch valves are generally recommended. Piping not properly inclined for drainability can have stagnant liquid in the system. Piping is recommended to be inclined at 0.5 to 1cm per meter of piping to facilitate drainability.
• Rugosity of surface: This is the surface roughness, where Ra – arithmetical mean roughness value. The arithmetical mean of the absolute values of the profile deviations from the mean line of the roughness profile. Corrosion on stainless steel surfaces leads to an increase in the surface roughness. When this occurs, the cleanability of the surface may be reduced. Because of this, the routine cleaning procedures may not be effective in removing residues from surfaces with increased roughness. Product residues left behind on a surface may provide a favorable condition for biofilm to grow.
• Residue: Important factors to consider when developing a cleaning cycle includes the use of appropriate cleaning agent, time, concentration of cleaning agent (if cleaning agent is used), and temperature. The cleaning cycle developed must be demonstrated to be consistently effective in the removal of product residues. Product residues left behind will affect sanitization or sterilization processes, and also residues left behind provide a nutrient source for microorganisms, especially if it is animal or plant-based.
Diagram 2: Factors leading to Biofilm Formation
Biofilm Remediation
One of the first signs of presence of biofilm in process equipment is the frequent occurrence of microbial excursions in microbiological test samples. The presence of self-produced EPS protects the microorganisms and increases their resistance to sanitization and cleaning. In the unfortunate event where a facility encounters recurring microbial contamination, the immediate tasks are to identify and eliminate the source of microbial contamination. A frequently asked question is, "How do we eliminate the biofilm ?"
Once there is a possibility of biofilm present in the system, a biofilm remediation procedure to both address the biofilm residues and sanitize the equipment should be performed. This is accomplished in three steps.
• Step 1: Use alkaline formulated cleaning chemistry to penetrate / denature the EPS.
The surfactants in the formulated alkaline cleaning chemistry will effectively penetrate and denature the EPS. It is critical that the protective layer of EPS is removed to ensure the success of the biofilm remediation.
• Step 2: Acid cleaning and passivation.
• When there is biofilm formation, it is also highly likely that the surface has some kind of corrosion in the form of rouge. Perform derouging and passivation in order to remediate the corroded surface. However, this step can be omitted if no rouge is observed.
• Step 3: Sanitization of system using sporicidal chemistry or steam.
This step eliminates the remaining microorganisms in the process systems through use of sporicidal chemistry such as a hydrogen peroxide and peracetic acid blend, isopropyl alcohol, ethanol, or steam sterilization.
The United States Environmental Protection Agency (EPA) has two test methods for evaluating the efficacy of antimicrobial germicides against two biofilm bacteria, Pseudomonas aeruginosa and Staphylococcus aureus, as well as regulatory guidance for label claims for those products. These test methods and guidance provide a framework for germicidal suppliers who wanted to have a biofilm removal claim on hard, non-porous surfaces using a broad-spectrum disinfectant.
• EPA MLB SOP MB-19: Growing a Biofilm using the CDC Biofilm Reactor
• EPA MLB SOP MB-20: Single Tube Method for Determining the Efficacy of Disinfectants against Bacterial Biofilm
The use of a broad-spectrum disinfectant with a biofilm removal claim is recommended in biofilm remediation strategy.
Preventive Measures
As the saying goes: "An ounce of prevention is worth a pound of cure." It is better to pro-actively prevent the formation of biofilm through the following considerations.
• Cleanability of the process equipment. Consider the engineering design of the process equipment to facilitate the cleaning process by having sanitary design and fittings.
• Robust cleaning with appropriate cleaning agents and cleaning parameters to effectively remove residues from the process equipment.
• Periodic stainless steel maintenance procedures by the use of effective acid based detergents.
• Optimal cleaning/sanitization frequency to maintain the equipment in a clean state.
• Routine trend analysis on the microbiological data collected enables system owners to know the state of microbial control of the process system and take early intervention if there is an adverse trend.
Conclusion
It is important to understand the lifecycle of biofilm, its characteristics, and remediation procedures. In addition, controlling bioburden in pharmaceutical, biotech, and medical device manufacturing processes is dependent on various factors. These factors include having a robust cleaning procedure, good engineering design, sound equipment maintenance program, and continuous monitoring and trending. These pro-active measures will help minimize the risk of microbial contamination in manufacturing facilities and ensures any drug products manufactured are safe for patients and consumers.
References
1) Pharmaceutical Technology Editors (Aug 22, 2018), King Bio Recalls Products Due to Microbial Contamination (http://www.pharmtech.com/king-bio-recalls-products-due-microbial-contamination)
2) Lopolito, P., and E. Rivera. "An Audit Approach to Address Microbial Contamination in Process Equipment." In Contamination Control in Healthcare Product Manufacturing, 2013, co-published by PDA and DHI
3) Cleanroom Technology, Strategies for Biofilm Remediation, A. Deal, D. Klein and P. Lopolito Jul 2015.
4) STERIS internal document, Tech Tip #3088 – General Cleaning Recommendation for Removal of Microbial Residue
About the Author
By Richard Chai
Technical Service Manager
STERIS Corporation
As a Technical Service Manager with STERIS Corporation based in Singapore, Richard has been providing technical training to customers in topics related to cleaning and bioburden control for cleanrooms, including disinfectant validation, as well as cleaning and cleaning validation for product contact surfaces. He is also an industry speaker at ISPE events. Prior to joining STERIS, Richard had 13 years of manufacturing and validation experience working in dry powder inhaler plant, biotech sterile fill and finish plant, and medical device and biopharmaceutical facilities. He has in-depth understanding of the requirements of cleaning and disinfection in cleanrooms, as well as best practices in bioburden control. Richard has also worked 3 years as a validation consultant providing validation support to various pharmaceutical customers in the areas of equipment validation, cleaning validation for product contact surfaces, disinfectant validation, as well as cleaning procedures for both product and non-product contact surfaces. If you need more information, please email: Richard_Chai@steris.com
Related Article:
Factors to Consider for a Cleaning and Disinfection Program
-----------------------------------------------------------------------
Editor's Note:
To become a freelance writer of En-CPhI.CN,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@ubmsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


