PharmaSources/XiaoyaowanAugust 27, 2019
Tag: Novartis , CAR-T , clinical application
Novartis filed a clinical trial application for CTL019 in China on Aug. 12, which has been accepted by the CDE. CTL019 is a breakthrough new CAR T-cell drug under Novartis, which is also the world’s first CAR T-cell therapy approved.
CTL019 Clinical Application Filed by Novartis in China | |||
Pharmaceutical product name | Acceptance No. | Registration class | Application type |
CTL019 | JXSL1900067 | Biological product for treatment | Import |
Source: Drug Clinical Trial Registration and Information Publicity Platform

The world’s first CAR T-cell therapy marketed
CAR T-cell therapies generally express the chimeric antigen receptors (CARs) on the surface of T cells and remove cancer cells using patients’ immune cells, being one of the most groundbreaking treatment means among oncology therapies at present.
Approved by the FDA for marketing on Aug. 30, 2017 with a trade name of Kymriah, Novartis’ CAR T-cell product: CTL019 is the first CAR T-cell therapy approved worldwide, which is used to treat relapsed/refractory acute lymphoblastic leukemia in children and young adults (2-25 years old) and priced at USD475,000.
Kymriah’s second indication was approved by the FDA on May 1, 2018 based on the previous Breakthrough Therapy Designation and corresponding clinical results, which is relapsed/refractory (r/r) diffuse large B-cell lymphoma (DLBCL) in adult patients.
Kymriah achieved global sales of USD76 million in 2018. Compared to the first generation, CTL019 therapy, the first therapy approved for marketing using the second-generation CAR T-cell technology, has additional intracellular costimulators which help strengthen the antitumor activity, with CAR T cells having stronger proliferation and persistence; it also has high stability and relatively mature process, being the mainstream technology at present.
According to the supportive clinical data, CTL019 showed extremely significant therapeutic effects in advanced patients with r/r blood tumor: its objective response rate and complete remission rate in pediatric patients with r/r acute lymphocytic leukemia (ALL) separately reached up to 95% and 83%.
Partial Clinical Trial Results of CTL019 | ||
Indication | r/r ALL in pediatric and young adult patients | DLBCL in adults |
Number of people | 63 | 68 |
Objective response rate (ORR) | 95% | 50% |
Complete remission rate | 83% | 32% |
Source: Organized according to public data
The competitor has striking performance and has taken the lead in the clinical trial in China
Kite Pharma’s KTE-C19 was approved by the FDA for marketing on Oct. 18, 2017, to become the world’s second CAR T-cell therapy approved for marketing, which is used to treat adult patients with a certain type of DLBCL, with the trade name: Yescarta and price of USD373,000.
Kite Pharma’s Yescarta and Novartis’ Kymriah are similar in terms of the structure. Yescarta achieved global sales of USD264 million in 2018, which were far higher than those of Novartis’ Kymriah. Besides, Yescarta has also been ahead of Novartis’ Kymriah in terms of the preparation to enter the Chinese market.
Kite Pharma and Fosun Pharma established a joint venture: Fosun Kite in China on Dec. 5, 2017 for the former to authorize commercialization rights and benefits of Yescarta in China to the latter.
Yescarta’s Clinical Trial Applied for by Fosun Kite in China | |||
Drug name | Registration No. | Indication | Trial professional title |
FKC876 | CTR20181687 | Relapsed/refractory large B-cell lymphoma | An Open-label Clinical Study to Evaluate the Safety and Effectiveness of FKC876 in Treating Relapsed/Refractory Aggressive Non-Hodgkin's Lymphoma (NHL) |
Source: Drug Clinical Trial Registration and Information Publicity Platform
Fosun Kite applied for a clinical trial for KTE-C19 in May 2018, to plan to recruit 15 subjects to evaluate the safety and effectiveness of the therapy in relapsed/refractory aggressive non-Hodgkin's Lymphoma (NHL), with the indication relapsed/refractory large B-cell lymphoma including the DLBCL, primary mediastinal large B-cell lymphoma (PMBCL), high grade B-cell lymphoma (HGBCL), and transformed follicular lymphoma (TFL). The clinical trial has enrolled the first subject so far.
The number of studies in Asia led by China ranks top in the world
The CDE has successively issued the Cell Product Study and Evaluation Technical Guidelines (Draft for Comment) and Cell Therapy Product Study and Evaluation Technical Guidelines (for Trial Implementation) since 2016, to make clear the general principles and basic requirements for cell therapy products in China from early R&D to production and from pharmaceutical research and non-clinical study stage to clinical study stage. The policies have boosted the development of Chinese-produced cell therapies and driven the industry to enter from chaos to stability.
There have been over 100 relevant companies and medical product suppliers in the Chinese cell therapy industry, with the influx of massive R&D funds. According to ClinicalTrials website, there have been 362 clinical studies conducted with chimeric receptors as the core to date, including 187 in East Asia led by China, which exceed those in the North America to rank top in the world.
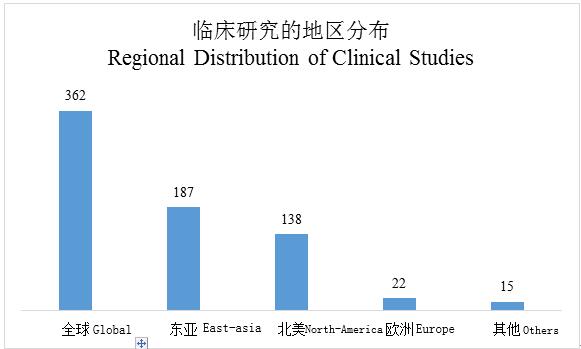
Source: ClinicalTrials
There has been no CAR T-cell therapy approved for marketing as a pharmaceutical product in China, however, the number of products approved for clinical trials is big, most of which target CD19. Many CAR T-cell products are expected to be marketed successively in China in the next 5 years.
Xiaoyaowan, a pharmaceutical industry practitioner, a word carrier in the We-media era focusing on changes of the pharma industry.
-----------------------------------------------------------------------
Editor's Note:
To become a freelance writer of En-CPhI.CN,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@ubmsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


