PharmaSources/DopineAugust 20, 2019
Tag: Breast Cancer , inhibitors , CDK 4/6
Targeted therapies have played an increasingly important role in tumor treatment in recent years. I’d like to introduce in detail an "upstart"—CDK 4/6 inhibitors in the breast cancer therapeutic area today. Pfizer’s palbociclib, Novartis’ ribociclib, and Eli Lilly’s abemaciclib have been approved for marketing and formed a situation of tripartite confrontation, wherein, the global sales of Pfizer’s palbociclib accounted for 89% of the CDK 4/6 inhibitor market in 2018 upon its first-mover advantage, however, the annual growth of palbociclib has gradually declined in recent years, and many enterprises have laid out the area. Therefore, it is still unknown as to whether which will dominate the CDK 4/6 inhibitor market in the future.

What are CDK 4/6 inhibitors?
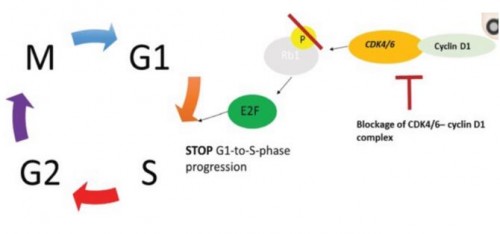
CDK, i.e., cyclin-dependent kinase, plays an important role in cell cycle control, wherein, CDK 4/6 can phosphorylate retinoblastoma (Rb) by binding to cyclin D, to release the transcription factor E2F to promote transcription of the cell cycle-related gene and make cells enter the S phase. According to research, in ER+ breast cancer, CDK 4/6 overactivity is very frequent, and CDK 4/6 is a key downstream target of ER signal. According to preclinical data, the dual inhibition of CDK 4/6 and ER signal has a synergy and can inhibit the growth of ER+ breast cancer cells in the G1 phase.
It is well known that unlimited proliferation and cell cycle deregulation are basic characteristics of tumors. Not only can CDK 4/6 inhibitors effectively block tumor cells from progressing from G1 phase to S phase, they can also inhibit the growth of G1-phase ER+ breast cancer cells, restore cell cycle control, and block tumor cell proliferation. On the other hand, the potential mechanism of action of CDK 4/6 inhibitors can also delay or overcome endocrine resistance and enhance the efficacy of endocrine therapy, which stands for the special advantages of CDK 4/6 inhibitors over other targeted drugs.
CDK 4/6 inhibitors, the "upstart" in the breast cancer therapeutic area in recent years, are rapidly changing the treatment patterns of HR+/HER2- advanced breast cancer; they can effectively overcome or delay the occurrence of endocrine resistance to strive for more survival time for advanced patients.
Pfizer’s palbociclib accounts for more than half of the market shares among the three CDK 4/6 inhibitors
Three CDK 4/6 inhibitors have been approved by the FDA for marketing, separately Pfizer’s palbociclib, Novartis’ ribociclib, and Eli Lilly’s abemaciclib.
As the first CDK 4/6 inhibitor approved by the FDA, palbociclib received the FDA’s accelerated approval in Feb. 2015 based on a Phase II study (PALOMA-1) to be used in combination with letrozole as a first-line therapy for postmenopausal ER+/HER2- advanced breast cancer. The application for expanding the indication of palbociclib—postmenopausal HR+/HER2- advanced breast cancer in patients who have failed the endocrine therapy in combination with fulvestrant was approved by the FDA in Feb. 2016 based on a randomized controlled Phase III study (PALOMA-3). Based on the results of a Phase III study: MONALEESA-2, ribociclib was approved by the FDA in Mar. 2017 as a first-line therapy for HR+ / HER2- advanced breast cancer in postmenopausal women. Abemaciclib, the third CDK 4/6 inhibitor approved, was approved to be used in combination with an aromatase inhibitor (AI) as a first-line therapy for postmenopausal HR+/HER2- advanced or metastatic breast cancer in Feb. 2018 based on the results of the MONARCH-3 study. And abemaciclib is the first and only CDK 4/6 inhibitor that can significantly prolong the lives of premenopausal/perimenopausal/postmenopausal patients in combination with fulvestrant. (See the following figure for the relevant clinical study information mentioned above.)
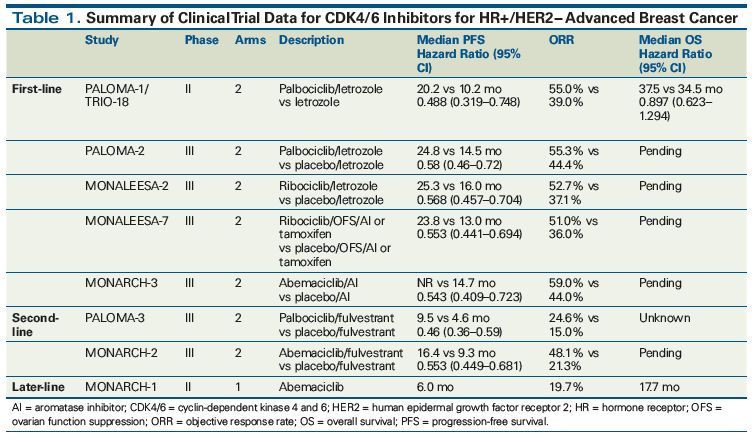
At ASCO 2018 Annual Meeting, Pfizer, Novartis, and Eli Lilly separately released the Phase III clinical trial data of their CDK 4/6 inhibitors used in HR+/HER2- breast cancer patients. According to the study data, the three CDK 4/6 inhibitors could significantly increase the objective response rate and prolong patients’ progression-free survival (PFS) no matter as first-line or second-line therapies or in premenopausal or postmenopausal patients. Especially as first-line therapies, the three CDK 4/6 inhibitors could all prolong the PFS for more than 10 months, while, as second-line therapies, the efficacy of fulvestrant±the three CDK 4/6 inhibitors was astonishingly consistent, with the hazard ratio all between 0.5 and 0.6, i.e., they could reduce the disease progression risk by about 40%-50%.
There have been no head-to-head clinical studies comparing the efficacy of the 3 drugs, however, compared to the data of endocrine monotherapy, the efficacy of the 3 CDK 4/6 inhibitors was comparable, only slightly different in terms of adverse reactions, for example, the main adverse reaction of palbociclib and ribociclib was granulocytopenia; the incidence of granulocytopenia caused by abemaciclib was relatively low, however, the incidence of diarrhea caused by abemaciclib was relatively high. Nonetheless, adverse reactions of the 3 selective CDK 4/6 inhibitors are still controllable and tolerable.
Pfizer’s palbociclib, the first CDK 4/6 inhibitor, achieved USD723 million sales in the first year of marketing, and its sales have continued to grow every year thereafter, therefore, it is a well-deserved super blockbuster, however, the annual growth of palbociclib has started to decline (see the following table) since the other two CDK 4/6 inhibitors were approved for marketing in 2017. According to the forecast of the U.S. EvaluatePharma, the annual sales of palbociclib are expected to reach USD8.2 billion by 2024. Palbociclib has been approved by the NMPA in Aug. 2018 and marketed in the trade name of Ibrance in China.
Trade name | 2015 sales | 2016 sales | 2017 sales | 2018 sales | 2016 growth | 2017 growth | 2018 growth |
Ibrance | 7.23 | 21.35 | 31.26 | 41.18 | 195.30% | 46.42% | 31.73% |
The situation of tripartite confrontation has been formed in the CDK 4/6 inhibitor market with the marketing of Novartis’ ribociclib and Eli Lilly’s abemaciclib. The two were marketed late but their annual growth was very high to separately reach 209.21% and 1,114.29%. Sales of ribociclib and abemaciclib will be clearly better as their indications are expanded, and they stand a good chance to increase respective market shares to challenge Pfizer’s palbociclib for ranking first in sales.
Trade name | 2015 | 2016 | 2017 | 2018 | 2016 | 2017 | 2018 |
Ibrance | 7.23 | 21.35 | 31.26 | 41.18 | 195.30% | 46.42% | 31.73% |
Kisqali |
|
| 0.76 | 2.35 |
|
| 209.21% |
Verzenio |
|
| 0.21 | 2.55 |
|
| 1,114.29% |
Unit of sales: USD100 million | |||||||
Studies and clinical applications of CDK 4/6 inhibitors have achieved great success, however, enterprises and wholesale medical supply companies are still studying applications of CDK 4/6 inhibitors in other breast cancer subtypes and other tumor areas. I believe that CDK 4/6 inhibitors will be applied more extensively in the near future, to not only benefit more cancer patients but also create huge economic benefits. However, which to dominate the CDK 4/6 inhibitor market remains to be seen.
References:
1. O'LearyB, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. NatRev Clin Oncol 2016;13: 417-30.
2. OttoT, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. NatRev Cancer 2017;17: 93-115.
3. OzakiA, Tanimoto T, Saji S. Palbociclib in Hormone-Receptor-Positive Advanced BreastCancer. N Engl J Med 2015;373: 1672-3.
4. SledgeGJ, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: Abemaciclib inCombination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer WhoHad Progressed While Receiving Endocrine Therapy. J Clin Oncol 2017;35:2875-84.
5. SlamonDJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Phase IIIRandomized Study of Ribociclib and Fulvestrant in Hormone Receptor-Positive,Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer:MONALEESA-3. J Clin Oncol 2018: O2018789909.
-----------------------------------------------------------------------
Editor's Note:
For any copyright disputes involving the content,
please email: Julia.Zhang@ubmsinoexpo.com


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


